Befintliga uppförandekoder för patientmedverkan med olika intressenter täcker inte hela omfattningen av forskning och utveckling (FoU). Europeiska läkemedelsmyndigheten (EMA) har sedan 2006 utvecklat ett omfattande ramverk för samverkan med patienter och konsumentorganisationer.
Den europeiska patientakademins (EUPATI) vägledningsdokument syftar till att stödja integreringen av patientmedverkan i hela processen för läkemedelsforskning och utveckling med tillsynsmyndigheter, organ för utvärdering av medicinsk teknik (HTA), etiska kommittéer och läkemedelsindustrin.
Användare kan avvika från vägledningen beroende på särskilda omständigheter, nationell lagstiftning eller de unika behoven i varje interaktion. Vägledningsdokumenten bör anpassas till individuella krav med hjälp av bästa professionella omdöme. Du kan läsa den redaktionella delen av vägledningen här.
EUPATI har publicerat fyra omfattande vägledningsdokument som behandlar patientmedverkan inom forskning och utveckling (FoU). Dessa dokument täcker viktiga aspekter, inklusive etiska kommittéer, organ för utvärdering av medicinsk teknik, regleringsprocesser och läkemedelsindustriledd forskning och utveckling av läkemedel.
Varje vägledningsdokument rekommenderar arbetsmetoder och processer och föreslår specifika aktiviteter och områden för patientmedverkan. I varje vägledningsdokument föreslås nyckelområden med möjligheter till patientmedverkan. De ger inte bara praktiska rekommendationer om hur man underlättar effektiva och transparenta interaktioner, utan ger också värdefulla insikter om vilka typer av patientinflytande som krävs. Detta omfattar individuella synpunkter som bygger på anekdotiska patientupplevelser, bidrag från patientföreträdare som representerar bredare patientbehov, formella ståndpunkter som förespråkas av patientorganisationer och synpunkter från patientexperter som, utöver sin erfarenhetsbaserade kunskap, har tekniska färdigheter och utbildning.
För att säkerställa att de är relevanta och i linje med föränderliga standarder och lagstiftning genomgår alla vägledande dokument regelbunden granskning och revidering, vilket återspeglar områdets dynamiska natur.
1. Vägledning för patientmedverkan i industriledd forskning och utveckling av läkemedel
EUPATI:s vägledande dokument i denna artikel syftar till att ge rekommendationer för grundregler och förslag för integrering av patientmedverkan i hela processen för forskning och utveckling av läkemedel inom läkemedelsindustrin och beskriver specifika aktiviteter där patienter kan involveras och påverka framtida forskning och utveckling av läkemedel.
2. Vägledning för patientmedverkan i HTA
Denna vägledning innehåller en uppsättning inledande ”övergripande principer” som är tillämpliga i hela processen för forskning och utveckling av läkemedel, vägledningens ansvarsfriskrivning, vägledningens omfattning, en förklaring av den definition av termen ”patient” som antagits av EUPATI, skälen till att utveckla vägledningen, bakgrundsinformation om patientmedverkan i HTA i Europa och de slutliga målen för vägledningen. Dessa avsnitt följs av rekommendationerna (föreslagna arbetsmetoder och aktiviteter för patientmedverkan).
3. Vägledning för patientmedverkan i regleringsprocesser
EUPATI-vägledningen i denna artikel omfattar patientmedverkan på det regulatoriska området. Den riktar sig främst till tillsynsmyndigheter som vill interagera med patienter eller deras organisationer i sin verksamhet, men bör också beaktas av patienter/patientorganisationer som planerar att samarbeta med tillsynsmyndigheter.
4. Vägledning för patientmedverkan vid etisk granskning av kliniska prövningar
Denna vägledning har tagits fram av European Patient Academy on Therapeutic Innovation (EUPATI) för alla intressenter inom läkemedelsutveckling som deltar i den etiska granskningen av kliniska forskningsprojekt, med särskild tonvikt på medlemmar i forskningsetiska kommittéer och patienter/vårdgivare eller patientrepresentanter som ger synpunkter från patienterna.
Dessa vägledningsdokument lägger en stark grund för patientmedverkan i de komplicerade processerna för forskning och utveckling av läkemedel och ger praktiska rekommendationer och insikter, medan följande färdplan för patientmedverkan syftar till att ytterligare stärka intressenterna att navigera dessa processer effektivt och erbjuder ett systematiskt ramverk för patientmedverkan under hela livscykeln för forskning och utveckling.
Färdplan för patientengagemang
Video om EUPATI:s färdplan för patientinvolvering i forskning och utveckling av läkemedel
Vad är EUPATI:s färdplan för patientengagemang?
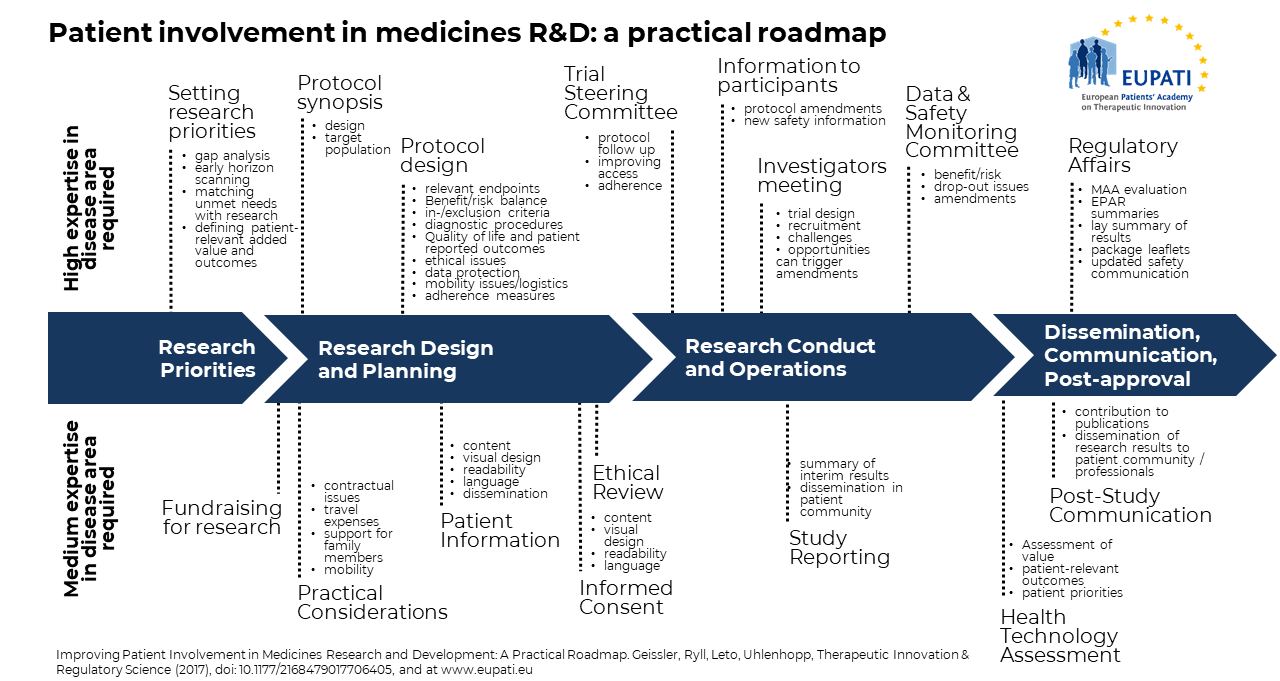
Värdet av patientmedverkan i forskning och utveckling av läkemedel erkänns alltmer av alla intressenter. Tyvärr hindrar begränsad formell dokumentation av aktiviteter för patientmedverkan utbyte av erfarenheter och lärdomar, vilket förhindrar snabb och systematisk implementering. Patientinvolvering saknar ofta struktur och enhetlighet i tillvägagångssättet och sker för sent. Det behövs en heltäckande, praktisk riktlinje och EUPATI Patient Engagement Roadmap, en processmodell för patientmedverkan i forskning och utveckling av läkemedel, tillhandahåller detta. Se bilden ovan (Källa: Geissler, J., Ryll, B., Leto di Priolo, S., Uhlenhopp, M.: Improving Patient Involvement in Medicines Research and Development: A Practical Roadmap. Therapeutic Innovation & Regulatory Science 2017).
Använda EUPATI:s färdplan för patientengagemang
Färdplanen belyser specifika möjligheter till patientmedverkan längs de fyra viktigaste stegen i livscykeln för forskning och utveckling av läkemedel och illustreras med konkreta exempel. Syftet med denna färdplan är att tillhandahålla ett verktyg för att underlätta patientmedverkan under denna livscykel och delas för att uppmuntra implementering och ytterligare förfining. Färdplanen avser att stimulera till ytterligare diskussion. Alla berörda parter, den akademiska världen och läkemedelsindustrin, patientorganisationer och patienter, kliniker och forskare, kommer att behöva delta i identifieringen av strategiska punkter för patientmedverkan och deras genomförande för att maximera fördelarna för alla intressenter.
EUPATI:s åtagande att genomföra färdplanen
Frågan om ett framgångsrikt genomförande är en viktig utmaning, eftersom färdplanen och vägledningsdokumenten måste omsättas i praktiken av de olika intressenterna. Att involvera patienter i forskning kan vara till stor nytta för läkemedelsutvecklingsprocessen: genom att föra in sina prioriteringar och perspektiv kan patienter bidra till att utveckla bättre behandlingar för dem själva och andra. Ett större patientdeltagande i FoU kommer att förbättra nya behandlingars effektivitet och säkerhet och öka allmänhetens stöd för medicinsk forskning. För att möjliggöra detta är det viktigt att patienterna har ingående kunskaper om de processer och metoder genom vilka läkemedel utvecklas och släpps ut på marknaden, så att de förstår var och hur de kan göra en meningsfull inverkan. EUPATI:s långsiktiga mål är fortfarande inriktade på rigorös utveckling av innehåll för patientutbildning, främjande av patientexperternas färdigheter i påverkansarbete och stärkandet av en europeisk patientrörelse. EUPATI breddar också sin verksamhet till att omfatta utbildning för andra intressenter, t.ex. utbildning i patientengagemang för personer som arbetar inom industrin och den akademiska världen.
[Friskrivningsklausul: Den översättning som visas har skapats med hjälp av ett automatiskt språkbehandlingssystem].