Existing codes of practice for patient involvement with various stakeholders do not comprehensively cover the full scope of research and development (R&D). The European Medicines Agency (EMA) has developed a comprehensive framework of interaction with patients and consumer organisations since 2006.
The European Patients’ Academy (EUPATI) guidance documents aim to support the integration of patient involvement across the entire process of medicines research and development with regulatory agencies, health technology assessment(HTA) bodies, ethics committees and the pharmaceutical industry.
Users may deviate from guidance according to specific circumstances, national legislation or the unique needs of each interaction. The guidance documents should be adapted for individual requirements using best professional judgment. You can read the editorial of the guidance here.
EUPATI has published four comprehensive Guidance Documents addressing patient involvement in the realm of research and development (R&D). These Documents cover key aspects, including ethics committees, health technology assessmentbodies, regulatory processes, and pharmaceutical industry-led medicines R&D.
Each Guidance Document recommends working methods and processes and suggests specific activities and areas for patient involvement. Each Guidance suggests key areas with opportunities for patient involvement. Not only do they provide practical recommendations on how to facilitate effective and transparent interactions, but also offer valuable insights into the types of patient input required. This encompasses individual input drawn from anecdotal patient experiences, contributions from patient advocates representing broader patient needs, formal positions advocated by patient organizations, and input from patient experts who, in addition to their experiential knowledge, possess technical skills and training.
To ensure their relevance and alignment with evolving standards and legislation, all Guidance Documents undergo periodic review and revision, reflecting the dynamic nature of the field.
1. Guidance for Patient Involvement in Industry-Led Medicines R&D
The EUPATI Guidance Document in this article aims at providing recommendations for ground rules and proposals for the integration of patient involvement across the entire process of medicines R&D in the pharmaceutical industry and outlines specific activities where patients can be involved and influence future medicines research and development.
2. Guidance for Patient Involvement in HTA
This Guidance includes a set of introductory “overarching principles” applicable throughout the medicines research and development process; the guidance disclaimer; the scope of the guidance; an explanation of the definition of the term “patient” adopted by EUPATI; the rationale for developing the guidance; background information about patient involvement in HTA in Europe; and the ultimate objectives of the guidance. These sections are followed by the recommendations (suggested working practices and patient involvement activities).
3. Guidance for Patient Involvement in Regulatory Processes
The EUPATI Guidance in this article covers patient involvement in the regulatory field. It is primarily aimed at regulatory authorities wishing to interact with patients or their organizations in their activities but should also be considered by patients/patient organizations planning to collaborate with regulatory authorities.
4. Guidance for Patient Involvement in Ethical Review of Clinical Trials
This Guidance has been developed by the European Patient Academy on Therapeutic Innovation (EUPATI) for all stakeholders in medicines development involved in the ethical review of clinical research projects, with special emphasis on members of research ethics committees and patients/carers or patient representatives providing patient input.
These Guidance Documents lay a strong foundation for patient involvement in the intricate processes of medicines R&D, ensuring practical recommendations and insights, while the following Patient Engagement Roadmap aims to further empower stakeholders to navigate these processes efficiently, offering a systematic framework for patient engagement throughout the R&D lifecycle.
Patient Engagement Roadmap
The EUPATI Patient Involvement in Medicines R&D Roadmap Video
What is the EUPATI Patient Engagement Roadmap?
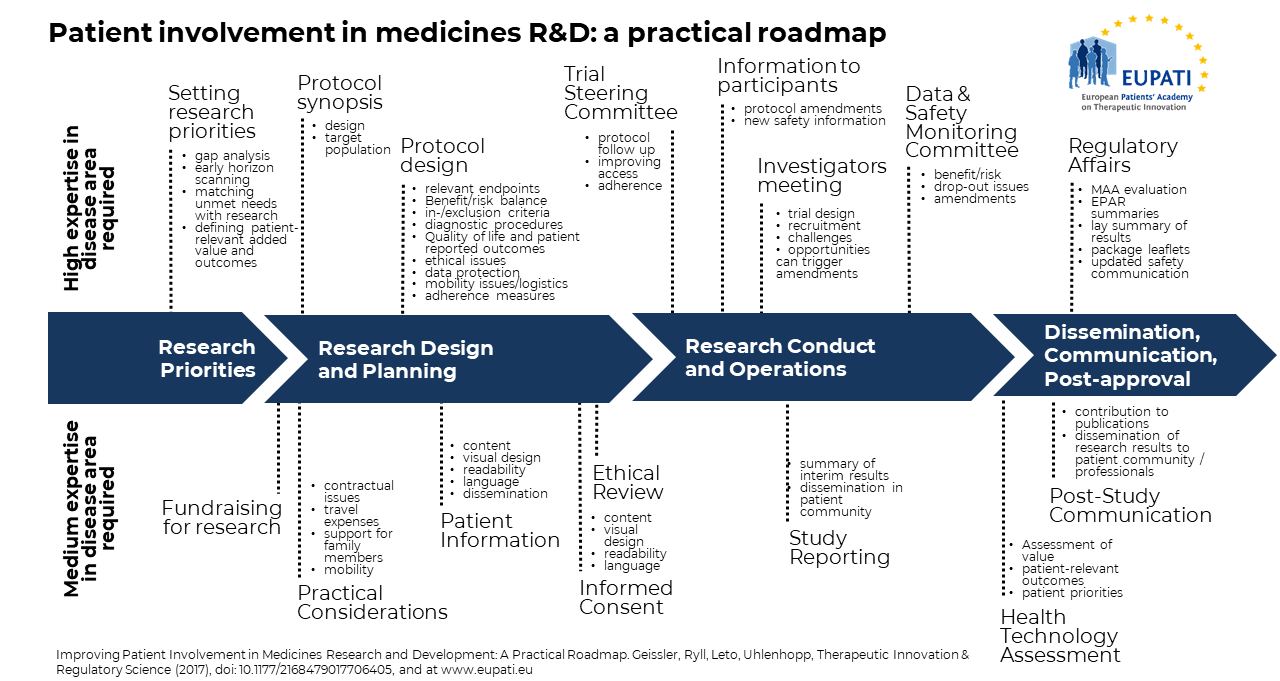
The value of patient involvement in medicines research and development is increasingly recognised by all stakeholders. Unfortunately, limited formal documentation of patient involvement activities hampers the sharing of experience and learnings, preventing timely and systematic implementation. Patient involvement often lacks structure and consistency in approach and happens too late. An end to end, practical guideline is required and the EUPATI Patient Engagement Roadmap, a process model for patient involvement in medicines Research and Development, provides this. Please see the image above (Source: Geissler, J., Ryll, B., Leto di Priolo, S., Uhlenhopp, M.: Improving Patient Involvement in Medicines Research and Development: A Practical Roadmap. Therapeutic Innovation & Regulatory Science 2017.)
Using the EUPATI Patient Engagement Roadmap
The roadmap highlights specific opportunities for patient involvement along the four key stages of the medicines Research and Development lifecycle and is illustrated with concrete examples. This roadmap’s aim is to provide a tool to facilitate patient involvement during this lifecycle and is being shared to encourage implementation and further refinement. The roadmap intends to stimulate further discussion. All involved parties, academia and pharmaceutical industry, patient organizations and patients, clinicians and researchers, will need to be involved in the identification of strategic patient involvement points and their implementation to maximize the benefit for all stakeholders.
EUPATI’s Commitment to the Implementation of the Roadmap
The question of successful implementation is an important challenge, as the Roadmap and the Guidance Documents need to be put into practice by the different stakeholders. Involving patients in research can hugely benefit the medicines development process: by bringing in their priorities and perspectives, patients can contribute to developing better treatments for them and others. Greater patient involvement in R&D will boost the efficacy and safety of new treatments and increase public support for medical research. To enable this, it is essential that patients have thorough knowledge of the processes and methods by which medicines are developed and brought to the market to understand where and how they can make a meaningful impact. EUPATI’s long-term objectives remain focused on rigorous content development for patient education, the furthering of advocacy skills of patient experts, and the strengthening of a European patient movement. EUPATI is also widening its scope of activities to include training towards other stakeholders, e.g. patient engagement training for professionals working in industry and academia.