Last update: 11 July 2023
Published and available for citation: Hunter A, Facey K, Thomas V, Haerry D, Warner K, Klingmann I, May M and See W (2018) EUPATI Guidance for Patient Involvement in Medicines Research and Development: Health Technology Assessment. Front. Med. 5:231. doi: 10.3389/fmed.2018.00231
Overarching principles for patient involvement throughout the medicines research and development process
The European Patients’ Academy (EUPATI) is a pan-European Innovative Medicines Initiative (IMI) project of 33 organisations with partners from patient organisations, universities, not-for-profit organisations, and pharmaceutical companies. Throughout EUPATI the term ‘patient’ references all age groups across conditions. EUPATI does not focus on disease-specific issues or therapies, but on process of medicines development in general. Indication-specific information, age-specific or specific medicine interventions are beyond the scope of EUPATI and are the remit of health professionals as well as patient organisations. To find out more visit eupati.eu/.
The great majority of experts involved in the development and evaluation of medicines are scientists working both in the private and public sector. There is an increasing need to draw on patient knowledge and experience in order to understand what it is like to live with a specific condition, how care is administered and the day-to-day use of medicines. This input helps to improve discovery, development, and evaluation of new effective medicines.
Structured interaction with patients of all age groups and across conditions, their representatives and other stakeholders is necessary and allows the exchange of information and constructive dialogue at national and European level where the views from users of medicines can and should be considered. It is important to take into account that healthcare systems as well as practices and legislation might differ.
We recommend close cooperation and partnership between the various stakeholders including healthcare professionals’ organisations, contract research organisations, patients’ and consumers’ organisations*, academia, scientific and academic societies, regulatory authorities and health technology assessment (HTA) bodies and the pharmaceutical industry. Experience to date demonstrates that the involvement of patients has resulted in increased transparency, trust and mutual respect between them and other stakeholders. It is acknowledged that the patients’ contribution to the discovery, development and evaluation of medicines enriches the quality of the evidence and opinion available.[1]
Existing codes of practice for patient involvement with various stakeholders do not comprehensively cover the full scope of research and development (R&D). The EUPATI guidance documents aim to support the integration of patient involvement across the entire process of medicines research and development.
These guidance documents are not intended to be prescriptive and will not give detailed step-by-step advice.
EUPATI has developed these guidance documents for all stakeholders aiming to interact with patients on medicines research and development (R&D). Users may deviate from this guidance according to specific circumstances, national legislation or the unique needs of each interaction. This guidance should be adapted for individual requirements using best professional judgment.
There are four separate guidance documents covering patient involvement in:
- Pharmaceutical industry-led medicines R&D
- Ethics committees
- Regulatory authorities
- Health technology assessment (HTA).
Each guidance suggests areas where at present there are opportunities for patient involvement. This guidance should be periodically reviewed and revised to reflect evolution.
This guidance covers patient involvement in health technology assessment (HTA).
All subsequently developed guidance should be aligned with existing national legislation covering interactions as stated in the four EUPATI guidance documents
Disclaimer
EUPATI has developed this guidance for all stakeholders aiming to interact with patients on medicines research and development (R&D) throughout the medicines R&D lifecycle.
These guidance documents are not intended to be prescriptive and will not give detailed step-by-step advice. This guidance should be used according to specific circumstances, national legislation or the unique needs of each interaction. This guidance should be adapted for individual requirements using best professional judgment.
Where this guidance offers advice on legal issues, it is not offered as a definitive legal interpretation and is not a substitute for formal legal advice. If formal advice is required, involved stakeholders should consult their respective legal department if available, or seek legal advice from competent sources.
EUPATI will in no event be responsible for any outcomes of any nature resulting from the use of this guidance.
The EUPATI project received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° 115334, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA companies.
Scope
This European guidance covers the interaction between Health Technology Assessment (HTA) bodies and patients in relation to medicines for human use. . “Patients” can be individual patients or their carers, or representatives from patient organisations with relevant expertise (section 4).
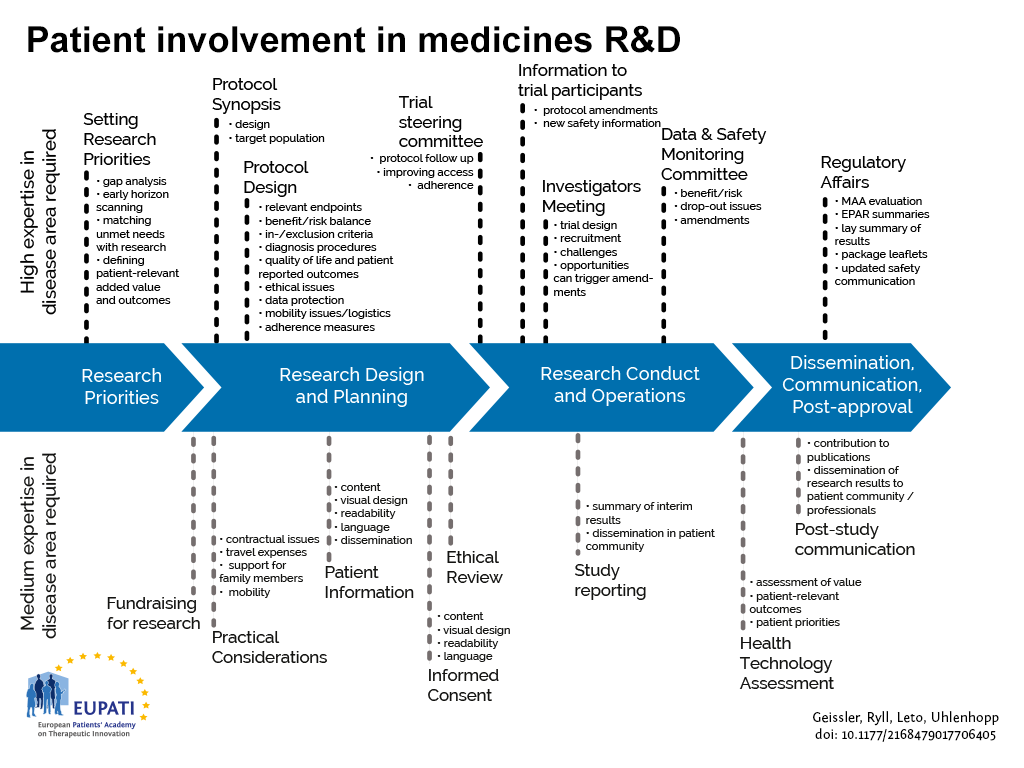
HTA processes are applied to interventions other than medicines, but they are not the focus of this guidance, in line with the remit of EUPATI. Figure 1 indicates where patients can be involved currently throughout the medicines R&D lifecycle; however this is not meant to limit involvement, and opportunities may change and increase over time.
The guidance focuses on participation in the HTA process, and excludes the scientific collection of patient perspectives (i.e. it excludes quantitative and qualitative research on the perspectives, experiences and preferences of patients).
The guidance draws together the outcomes of several research and consensus-building processes carried out by a variety of national and international organisations. It also draws on good practice examples from individual HTA agencies. These sources are referenced as they arise in the guidance.
- Patients can be involved across the process of medicines R&D. This diagram created by Geissler, Ryll, Leto, and Uhlenhopp identifies some existing areas in which patients are involved in the process. It distinguishes between the level of expertise in a disease area that is required and the different areas where involvement can take place.
Defining “patient”
The term “patient” is often used as a general, imprecise term that does not reflect the different types of input and experience required from patients, patient advocates and patient organisations in different collaborative processes.
In order to clarify terminology for potential roles of patient interaction presented in this and the other EUPATI guidance documents, we use the term “patient” which covers the following definitions:
- “Individual Patients” are persons with personal experience of living with a disease. They may or may not have technical knowledge in R&D or regulatory processes, but their main role is to contribute with their subjective disease and treatment experience.
- “Carers” are persons supporting individual patients such as family members as well as paid or volunteer helpers.
- “Patient Advocates” are persons who have the insight and experience in supporting a larger population of patients living with a specific disease. They may or may not be affiliated with an organisation.
- “Patient Organisation Representatives” are persons who are mandated to represent and express the collective views of a patient organisation on a specific issue or disease area.
- “Patient Experts”, in addition to disease-specific expertise, have the technical knowledge in R&D and/or regulatory affairs through training or experience, for example EUPATI Fellows who have been trained by EUPATI on the full spectrum of medicines R&D.
There may be reservations about involving individual patients in collaborative activities with stakeholders on grounds that their input will be subjective and open to criticism. However, EUPATI, in line with regulatory authorities, instils the value of equity by not excluding the involvement of individuals. It should be left to the discretion of the organisation(s) initiating the interaction to choose the most adequate patient representation in terms of which type of patient for which activity (see section 7.1). Where an individual patient will be engaged it is suggested that the relevant patient organisation, where one exists, be informed and/or consulted to provide support and/or advice.
The type of input and mandate of the involved person should be agreed in any collaborative process prior to engagement.
Rationale for the guidance
HTA stands for Health Technology Assessment. The main aim of HTA is to inform decision making by health care policy makers. It is a systematic process that considers health technologies (such as medicines or medical devices) and can involve a review of:
- clinical effectiveness (how well a medicine will work in the local health system compared to the best standard of care)
- cost effectiveness (the long term costs and benefits of a medicine compared to the best standard of care)
- social and ethical impacts on the health care system and the lives of individual patients.
The process advises whether or not a health technology should be used, and if so, how it is best used and which patients are most likely to benefit from it. Assessments vary, but most look at the health benefits and risks of using the technology. They can also look at costs and any other wider impacts that the technology may have on a population or on a society.[2]
HTA assesses international evidence but applies it to the local health care setting to understand the added value of a new medicine in that health care system. HTAs are performed at national, regional or hospital level.
The importance of patient involvement in HTA is becoming widely recognised. Patients are directly affected by HTA decisions - they are key stakeholders, and have a “democratic right” to be involved.[3] HTA can be considered to be a bridge between scientific evidence and decision-making[4] and as a result there are both scientific and democratic reasons that support effective patient involvement in HTA.
Patients can provide information and insight, about the impact of their condition and treatments on their daily lives that is not available elsewhere. Patients are in a unique position to describe the outcomes that matter to them, to challenge presumptions about their health aspirations and to inform HTA processes about the potential positive or negative effects of new and existing technologies - on their health and on their ability to live and work.
Introduction
The extent of patient involvement in HTA varies considerably between countries and regions in Europe. Commonly HTA is still focused on quantitative evidence to determine clinical and/or cost effectiveness, although there are instances of active patient support. [5][6][7]
The extent and nature of support for patients provided by HTA bodies, to optimise patient involvement in their processes, also varies a great deal.[2][6][8]
The involvement of patients in HTA is determined at the national and regional level, and is not subject to any European legislation.
HTA bodies and patient organisations have reported a positive impact of patient involvement on the processes and/or outcomes of HTA. Although systematic research into the impact of different approaches of patient involvement is scarce[7][9][10] those case studies that are available make the impact of patient involvement explicit. Bodies such as HTAi and ISPOR are working to develop the evidence base and provide repositories of materials for patient involvement.[11][12]
The HTA Core Model® (version 3.0) produced by EUnetHTA[13] (a network of government appointed organisations, regional agencies and non-for-profit organisations that produce or contribute to HTA in Europe) provides a detailed technical guideline for HTA agencies, outlining the types and sources of evidence required for HTA. Patients are included as potential sources of evidence. The HTA Core Model® is aimed at professionals with HTA expertise and the topic of patient involvement in HTA processes more widely is outside its scope.
There is therefore a need for a Europe-wide guidance on patient interaction in HTA to promote good practice and to complement the work of EUnetHTA.
Objectives of the guidance
The following values are recognised in the guidance, and worked towards through the adoption of the suggested working practices (section 7). The values, given in the table below, are one output of a consensus-building exercise by HTAi. Patient organisations, academia, HTA agencies and industry contributed to the exercise, which received input from 150 respondents in 39 countries.[14]
The values are:
| Relevance | Patients have knowledge, perspectives and experiences that are unique and contribute to essential evidence for HTA. |
| Fairness | Patients have the same rights to contribute to the HTA process as other stakeholders and have access to processes that enable effective engagement. |
| Equity | Patient involvement in HTA contributes to equity by seeking to understand the diverse needs of patients with particular health issues, balanced against the requirements of a health system that seeks to distribute resources fairly among all users. |
| Legitimacy | Patient involvement facilitates those affected by the HTA recommendations/decision to participate in HTA; contributing to the transparency, accountability and credibility of the decision-making process. |
| Capacity building | Patient involvement processes address barriers to involving patients in HTA and build capacity for patients and HTA organisations to work together. |
Suggested working practices
The working methods recommended for HTA agencies and patient organisations in this section arise from several sources. The primary sources are the set of quality standards from the HTAi consensus-building exercise, reviews of individual HTA agencies and the EPF survey of patient involvement in HTA in Europe.[13][15][16][17][18][19][20] Specific patient involvement activities that are employed or planned by HTA agencies are given in section 8 “Suggested patient involvement activities”.
For HTA bodies that are new to patient involvement, a step-wise approach to introducing new working methods and activities may be the most successful. Prioritisation of specific working methods and activities should be decided on by individual HTA bodies with patients and other stakeholders.
In order to achieve the objectives identified under section 6, the following should be considered by HTA bodies:
- should have a strategy that outlines the processes and responsibilities for those working in HTA and serving on HTA committees, to effectively involve patients.
- should designate appropriate resources to ensure and support effective patient involvement in HTA.
- HTA participants (including researchers, staff, HTA reviewers and committee members) should receive training about appropriate involvement of patients and consideration of patients’ perspectives throughout the HTA process.
- patients should be given the opportunity to receive mentoring and training so that they can contribute most effectively to HTA.
- patient involvement processes in HTA should be regularly reflected on and reviewed, taking account of the experiences of all those involved, with the intent to continuously improve the processes.
- should work to align internal and external stakeholders on the objectives of patient input processes.[18]
- should have proactive communications strategies to effectively reach, inform and enable a wide range of patients to participate fully in each HTA, including making public the criteria and processes they use to reach decisions.
- should have clear timelines established for each HTA with advance notice of deadlines to ensure that appropriate input from a wide range of patients can be obtained.
- for each HTA, should identify a staff member whose role is to support patients to contribute effectively to HTA.
- in each HTA, patients’ perspectives and experiences should be documented and the influence of patient contributions on conclusions and decisions should be reported.
- should provide feedback to patients who have contributed to an HTA, to share what contributions were most helpful and provide suggestions to assist their future involvement.
- each HTA should use accessible language in documents and other materials for the patients involved. [16]
- should give patients the opportunity to participate other than through making submissions◊ to specific HTAs.[18]
- should develop frameworks to systematically incorporate patient input to HTAs. [18]
- should make systems for written submissions easy to use and appropriate support should be offered to individuals making submissions. [16]
In order to achieve the objectives identified under section 6, the following should be considered by patient organisations:
- ensure those speaking on your behalf are trained in the nature of HTA, both its role in healthcare resource allocation and scientific and cost-effectiveness aspects.
- where there are no or few patient involvement activities, approach HTA agencies pro-actively and suggest how patient involvement can be achieved through clear proposals.
- understand the HTA processes: meet with HTA staff, follow guidelines and deadlines, use glossaries if available.
- learn from the experience of other patient organisations and collaborate with them.
- remain transparent: declare (publish) and diversify your financial support, and have a clear and explicit framework for cooperating with industry.
Suggested patient involvement activities
The suggested activities outlined in this section are examples of specific mechanisms to involve patients. They are all already practised (or planned) by one or more HTA bodies. They are drawn from publications from HTAi, EPF, INAHTA, individual HTA agencies and academic reviews. [5[6][7][16][17][18][21]
The following text uses the term “patient” to refer to the different categories defined on section 4.
General HTA process
Aimed at HTA organisations, the activities listed here will help implement the recommended working methods for the HTA process in general. The list does not aim to be exhaustive but to provide initial ideas.
Outreach and education
- produce guidance materials on the different roles patients may take within HTA processes.
- provide a single point of contact for patient involvement issues.
- give presentations and training workshops for patient organisation representatives, about HTA and patient involvement.
- evaluate and communicate about the impact patients have had to demonstrate that they can make a difference.
- hold HTA meetings in public as far as possible.
- provide a glossary in relevant language(s) of HTA-specific terms.
- advertise forthcoming HTAs including alerting through regular bulletins, and actively invite patient organisations to take part.
- support the development of peer support groups for patients involved with individual HTA bodies.
Wider involvement
- include patients when consulting on potentially significant changes to HTA processes.
- consider the use of participatory approaches, such as Citizen’s Jury[22] or consensus conference methods, during development of HTA processes.
- include patient experts as lay members, or in addition to lay members, of HTA committees not just as contributors to specific HTAs. Give these members full voting rights.
For individual HTAs
The activities listed here are again aimed at HTA organisations, to help implement the recommended working methods for individual HTAs. The list does not aim to be exhaustive but to provide initial ideas.
Identifying and prioritising which technologies to assess.
- develop a system for patients to nominate technologies for HTA.
Scoping (developing a framework for an individual HTA)
- consult with patient organisations on the draft scope using templates for written submissions.
- invite patient organisations to oral consultation meetings to take part in discussion on the HTA scope.
Assessing and developing recommendations/guidelines
- invite patient organisations to nominate patient and clinical experts to attend HTA committee meetings.
- invite written submissions from individual patients/carers and patient organisations to form part of the evidence base considered by the committee.
- provide templates, guidance documents and telephone support for those completing written submissions, and preparing to act as patient experts at meetings.
- invite oral submissions from individual patients/carers at committee meetings i.e. personal testimony.
- provide easy to read summaries of documentation sent out ahead of individual HTAs.
- give free access for patients to any original publications that will form part of the HTA evidence.
- develop an exit questionnaire for patients attending meetings, to be issued after each HTA, and feed results into the overall review of patient involvement.
Reviewing and disseminating HTA outcomes
- summarise patient input in HTA outcome documents, and how it was used in reaching the final recommendation. When suggestions from patients were not included in the final recommendation, provide a properly justified written explanation.
- provide lay language versions of HTA outcome documents.
- invite written comment on drafts of HTA outcomes from patients taking part in the HTA, and from others who were unable to take part (for example for health reasons).
- develop and disseminate a clear system for patients to appeal HTA decisions.
- involve patients in the review of patient involvement processes.
Compensation
It should be recognised that in many situations patients involved in activities do so voluntarily either as an individual but also when a member of an organisation. Consideration should therefore be given to:
- compensate for their total time invested plus expenses.
- any compensation offered should be fair and appropriate for the type of engagement. Ideally travel costs would be paid directly by the organising partner, rather than being reimbursed.
- covering the costs incurred by patient organizations when identifying or supporting patients for involvement in activities (i.e peer support groups, training and preparation) should also be considered.
- help organise the logistics of patient participation, including travel and/or accommodation.
Compensation also includes indirect benefits in kind (such as the a patient organisation providing services free of charge) or any other non-financial benefits in kind provided to the patient/patient organisation (such as training sessions, agency services, the setting up of web sites).
All parties should be transparent about any compensation arrangements.
Written agreement
At a minimum a written agreement should clearly define: a description of the activity and its objectives, the nature of the interaction during the activity, consent (if relevant), release, confidentiality, compensation, data privacy, compliance, declaration of conflict of interest, timelines. Interaction may only proceed on the basis of a written agreement that at a minimum spells out the basic elements of the collaboration (e.g., rules of engagement, compliance, intellectual property, financial payments).
Care should be taken so that written agreements are clear and do not limit appropriate knowledge sharing.
Appendix 1 Resources
International and country-specific resources for HTA agencies and patients
| International | |||
| Author | Resource | Date | Notes |
| Recommendations | |||
| HTAi | Values and Quality Standards for Patient Involvement in HTA
|
2014 | Outcome of international 18-month consensus building project. |
| EPF | Patient involvement in HTA in Europe. Results of the EPF survey.
http://www.eu-patient.eu/globalassets/projects/hta/hta-epf-final-report2013.pdf |
2013 | Includes recommendations for HTA bodies and for patient organisations. |
| Good practice examples | |||
| HTAi | Good Practice Examples of Patient and Public Involvement in Health Technology Assessment
https://htai.org/wp-content/uploads/2018/02/Good_Practice_Examples_Feb_2015.pdf |
2015 | Summary of approaches taken by HTA bodies, including several EU countries/regions. Includes some hints and tips for others. |
| Patient templates | |||
| HTAi | Patient group submission template for HTA of medicines
Follow link from: https://htai.org/interest-groups/pcig/resources/for-patients-and-patient-groups/
|
2014 | Includes brief guidance for patient groups.
|
| International | |||
| Author | Resource | Date | Notes |
| Patient templates cont. | |||
| Patient group submission template for HTA of health interventions (not medicines)
Follow link from: https://htai.org/interest-groups/pcig/resources/for-patients-and-patient-groups/
|
2015 | Includes brief guidance for patient groups. | |
| Completing a patient group submission template: guidance for patient organisations.
https://htai.org/wp-content/uploads/2018/02/PCISG-Resource-GuidanceandChecklist-Dec14.pdf |
2014 | For HTA bodies to adapt to their needs and those of their community. | |
| Education aimed at patients | |||
| EUPATI | EUPATI Patient Expert Training Course
Toolbox for education in medicines development. |
2015 | The training course takes new trainees each September/ October.
The training course and the online toolbox include HTA educational material for patients and patient representatives. |
| EURORDIS | Webcasts and slide presentations from annual summer school: | Includes a session on HTA. | |
| HTAi | Introducing HTA to patients and patient organisations.
|
2013 | Video and slide presentation.
|
| HEE | Understanding health technology assessment.
https://htai.org/wp-content/uploads/2018/02/PCISG-Resource-HEE_ENGLISH_PatientGuidetoHTA_Jun14.pdf |
2008 | Booklet available in English, Spanish, Mandarin, Italian, Polish, Swedish, Greek |
| International | |||
| Author | Resource | Date | Notes |
| Education aimed at other stakeholders (not patients) | |||
| EUnetHTA | Training for stakeholders.
|
This web link includes information as it becomes available. | |
| ISPOR | HTA training programme:
https://www.ispor.org/conferences-education/education-training |
||
| ISPOR | Regional Chapters’ activities:
|
Most regional ISPOR chapters are national. They provide a range of training and education opportunities. | |
| Contact information for HTA bodies | |||
| HTAi | List of HTA bodies worldwide:
http://vortal.htai.org/?q=about/producers_and_networks
|
||
| INAHTA | Contact information for HTA bodies worldwide:
|
||
| Glossaries | |||
| INAHTA/
HTAi |
Glossary of HTA terms:
|
Web-based glossary available in English, French, Spanish and German.
Updated periodically. |
|
| HTAi | HTAi consumer and patient glossary:
|
2009 | Glossary (pdf). Available in English and Greek. |
| International | |||
| Author | Resource | Date | Notes |
| Surveys | |||
| EPF | Patient involvement in HTA in Europe. An interim report on EPF's survey with HTA agencies in Europe.
|
2011 | |
| EPF | Patient involvement in HTA in Europe. An interim report on EPF's survey with decision makers in Europe.
http://www.eu-patient.eu/globalassets/projects/hta/report-hta-survey_decision-makers_final.pdf
|
2011 | |
| EPF | Patient involvement in HTA in Europe. An interim report on EPF's survey with patient organisations across Europe.
http://www.eu-patient.eu/globalassets/projects/hta/epf-report_hta-survey_po.pdf
|
2011 | |
| INAHTA | Involvement of consumers in the HTA activities of INAHTA members.
http://www.inahta.org/wp-content/uploads/2014/04/INAHTA_Survey_Consumer-Involvement_2011.pdf
|
2011 | Comparison of repeat survey of HTA agencies in 2005 and 2010. |
| Industry reports | |||
| Deloitte / Eli Lilly | Enhancing Consumer Involvement in Medicines Health Technology Assessment.
|
2009 | |
| Regional templates and guides, available online (Europe) | |||
| Body | URL or document title | Notes | |
| INVOLVE
(UK) |
Payment and recognition for public involvement.
http://www.invo.org.uk/resource-centre/payment-and-recognition-for-public-involvement/ |
Written for patient involvement in general research, but contains useful pointers for payment and recognition generally. | |
| IQTIG
(Germany) |
http://www.iqtig.de | Legal text and guidelines for patient participation in HTA. | |
| NICE
(England) |
http://www.nice.org.uk/about/nice-communities/public-involvement/develop-NICE-guidance | Comprehensive set of templates, factsheets and guides for patients/carers and patient organisations. | |
| NICE
(England) |
Confidentiality agreement for stakeholders taking part in HTAs.
https://www.nice.org.uk/get-involved/stakeholder-registration/confidentiality-agreement |
||
| SMC (Scotland) | http://www.scottishmedicines.org.uk/Public_Involvement/ | Comprehensive guidance, educational videos, Patient Group Partner Registration Form and Patient Group submission form. | |
| Evaluations of individual HTA bodies | |||
| Body | URL or document title | Notes | |
| CADTH
(Canada) |
CADTH Patient Input Process Review. Findings and Recommendations
https://www.cadth.ca/sites/default/files/pdf/2012_SECOR_Patient-Input-Review_e.pdf
|
Review published 2012. | |
| NICE
(England) |
Technology Appraisal Patient Expert Survey 2012 Report
|
Survey of patient involvement in NICE HTAs. | |
| Evaluations of individual HTA bodies | |||
| SMC
(Scotland) |
2014 Review of SMC Public Involvement
https://www.scottishmedicines.org.uk/Public_Involvement/Our_commitment_to_continuous_improvement
The Scottish Medicines Consortium and public attitudes to the provision of medicines for the NHS in Scotland. 2015
|
||
| Case studies of patient involvement | |||
| Body | Document title | Notes | |
| NICE (England) / SMC (Scotland) | Patient involvement in NICE technology appraisals. | Authored by Amis L. In “Patients, the public, and priorities in healthcare”. Edited by Peter Littlejohns and Michael Rawlins. Oxford: Radcliffe. 2009. | |
Appendix 2 Abbreviations
AOTM Agency for Health Technology Assessment (Poland)
AQuAS Agency for Health Quality and Assessment of Catalonia
CEDIT Hospital based Health Technology Assessment Agency (Paris, France)
EPF European Patients Forum
EUnetHTA European network for Health Technology Assessment
EUPATI European Patients’ Academy on Therapeutic Innovation
FOPH Federal Office of Public Health (Switzerland)
GBA Federal Joint Committee (Germany)
HTA Health Technology Assessment
HTAi Health Technology Assessment international
IQTIG Institute for Quality Assurance and Transparency in Healthcare (Germany)
ISPOR International Society for Pharmacoeconomics and Outcomes Research
IQWiG German Institute of Quality and Efficiency in Healthcare
ISPOR International Society for Pharmacoeconomics and Outcomes Research
NICE National Institute for Health and Care Excellence (England)
Osteba Basque Office for Health Technology Assessment
SBU Swedish Council for Technology Assessment
SMC Scottish Medicines Consortium
References
- Adapted from the EMA framework. European Medicines Agency (2022). EMA/649909/2021 Adopted. https://www.ema.europa.eu/en/documents/other/engagement-framework-european-medicines-agency-and-patients-consumers-and-their-organisations_en.pdf. Last Accessed 12 February 2024.
- HTAi (2015) “Completing a patient group submission template: guidance for patient organisations”. https://htai.org/interest-groups/pcig/resources/for-patients-and-patient-groups/. Last Accessed 4 July 2021.
- HTAi (2010) “Patients’ perspectives in health technology assessment: A route to robust evidence and fair deliberation.” International Journal of Technology Assessment in Health Care.
- Gauvin F-P et al. (2010) ”It all depends”: Conceptualizing public involvement in the context of health technology assessment agencies”. Social Science and Medicine.
- HTAi (2010 ) “Patients’ perspectives in health technology assessment: A route to robust evidence and fair deliberation.” International Journal of Technology Assessment in Health Care.
- EPF (2013) “Patient Involvement in Health Technology Assessment in Europe. Results of the EPF Survey.” http://www.eu-patient.eu/globalassets/projects/hta/hta-epf-final-report2013.pdf. Last Accessed 4 July 2021.
- INAHTA (2011) “Involvement of consumers in the HTA activities of INAHTA members. Report on a survey.”. http://www.inahta.org/wp-content/uploads/2014/04/INAHTA_Survey_Consumer-Involvement_2011.pdf. Last Accessed 4 July 2021.
- HTAi (2014) “Good practice examples of Patient and Public Involvement in Health Technology Assessment.” https://htai.org/wp-content/uploads/2018/02/Good_Practice_Examples_Feb_2015.pdf. Last Accessed 4 July 2021.
- Menon D, Stafinski T. (2011) “Role of patient and public participation in health technology assessment and coverage decisions.” Expert review pharmacoeconomics and outcomes research.
- Gagnon M-P et al. (2014) “Involving patients in the early stages of health technology assessment (HTA): a study protocol.” BMC Health Services Research.
- https://htai.org/wp-content/uploads/2018/02/Good_Practice_Examples_Feb_2015.pdf. Last Accessed 4 July 2021.
- http://www.ispor.org/sigs/PatientCentered/PC_EngagementInResearch.aspx. Last Accessed 17.03.2024.
- EUnetHTA Joint Action 2, Work Package 8. HTA Core Model ® version 3.0 (Pdf); 2015. Available from http://www.corehta.info/BrowseModel.aspx. Last Accessed 4 March 2024.
- HTAi (2014) “Values and Quality Standards for Patient Involvement in HTA.” https://htai.org/interest-groups/pcig/values-and-standards/. Last Accessed 17 July 2021.
- EPF (2013) “Patient Involvement in Health Technology Assessment in Europe. Results of the EPF survey.” http://www.eu-patient.eu/globalassets/projects/hta/hta-epf-final-report2013.pdf. Last Accessed 4 July 2021.
- NICE (2014) “Technology Appraisal Patient Expert Survey 2012 Report.” https://www.nice.org.uk/media/default/About/NICE-Communities/Public-involvement/Public-involvement-programme/Patient-expert-TA-report-final-1.pdf. Last Accessed 4 July 2021.
- SMC & Scottish Health Council (2015) “The Scottish Medicines Consortium and public attitudes to the provision of medicines for the NHS in Scotland”. http://www.scottishhealthcouncil.org/publications/gathering_public_views/idoc.ashx?docid=0b031cb1-17b4-482b-9f51-fd118e3cf541&version=-1. Last Accessed 4 July 2021.
- SECOR (2012) “CADTH Patient Input Process Review.” https://www.cadth.ca/sites/default/files/pdf/2012_SECOR_Patient-Input-Review_e.pdf. Last Accessed 4 July 2021.
- Australian Government Department of Health and Ageing (2009) “Review of health technology assessment in Australia.” http://www.health.gov.au/internet/main/publishing.nsf/Content/AF68234CE9EB8A78CA257BF00018CBEB/$File/hta-review-report.pdf. Last Accessed 21.11.2016.
- Messina J, Grainger DL. (2012) “A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. “ The Patient.
- Citizen’s Jury method is explained at The University of Manchester. (2012) https://www.methods.manchester.ac.uk/themes/data-collection/citizens-juries/, Last Accessed 5 July 2021.
*Consumers are recognised as stakeholders in the healthcare dialogue. The scope of EUPATI focuses on patients rather than consumers this is reflected in the educational material and guidance documents.