Mini-course Starter Kit – Protocol design
Introduction
This EUPATI Mini-course starter kit is designed for patient involvement in protocol design.
EUPATI Mini-course starter kits have been derived from content found in the EUPATI toolbox and EUPATI Patient Expert Training Course. The starter kits are thought to address roles that patients play in medicines development for example those shown in the figure below.
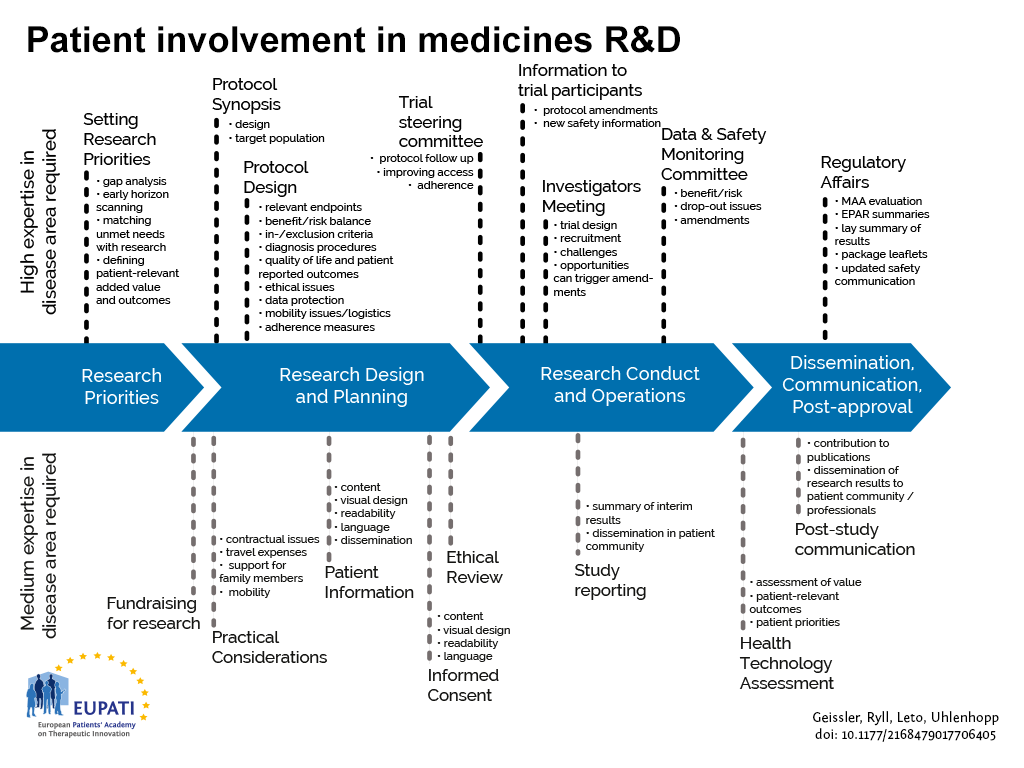
- Patients can be involved across the process of medicines R&D. This diagram created by Geissler, Ryll, Leto, and Uhlenhopp identifies some existing areas in which patients are involved in the process. It distinguishes between the level of expertise in a disease area that is required and the different areas where involvement can take place.
The starter kits provide you with links to relevant background reading in the toolbox and associated PowerPoint slide decks and media in order to prepare a single or multi-day training on the subject. Each of the starter kits contains a selection of PPT slides which you may use to educate patients/advocates about the “basics” in that area, e.g. in a two-hour to one-day seminar.
The starter kits are based on existing content from the EUPATI Toolbox, plus additional links to add-on Toolbox material. None of the “starter kits” are “ready-made course” modules – they are a ready-to-reuse resource for an experienced trainer to prepare and execute a course. You will need to edit them and put them into context.
Before you begin please download and review the ‘Manual for Trainers’: A manual for trainers describing how to use the EUPATI mini-course starter kits to create trainings on patient involvement.
[glossary_exclude]Protocol Design
This starter kit provides background reading, slides, a video, and quizzes to create training for patients who intend to become involved in protocol design.[/glossary_exclude]
[glossary_exclude]
Core reading
Making a medicine. Step 1: Pre-discovery
Making a medicine. Step 2: Target selection
Making a medicine. Step 3 and 4: Choosing a molecule or lead
Making a medicine. Step 5: Non-clinical safety testing
Making a medicine. Step 6: Phase I – Proof of mechanism
Making a medicine. Step 7: Phase II – Proof of concept
Making a medicine. Step 8: Confirmatory studies
Making a medicine. Step 9: Regulatory submission
Making a medicine. Step 10: Life-cycle management
New research areas in personalised medicines
Role of statistics in clinical trials
The concept of blinding in clinical trials
Data collection in clinical trials
Optional
Clinical trials in small populations
Paediatric medicine: Paediatric Investigation Plan
Patient reported outcomes (PROs) assessment
Basics of Early Clinical Development
Types of Study in Early Clinical Development
Presentations
Early clinical development (coming soon)
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
- Presentation: Clinical Trial Designs
Size: 1,179,726 bytes, Format: .pptx
A presentation covering the various types of clinical trial design. Details are given about blinding, control, comparisons, and randomisation.
- Presentation: Evidence-based Medicine
Size: 454,790 bytes, Format: .pptx
A presentation describing evidence based medicine, which can be adapted for own use.
- Presentation: The Role of Statistics in Clinical Trials
Size: 354,126 bytes, Format: .pptx
A presentation describing the role of statistics in clinical trials, which can be adapted for own use.
- Presentation: Statistics in Clinical Trials: Key Concepts
Size: 381,778 bytes, Format: .pptx
A presentation describing the key concepts of statistics in clinical trials, which can be adapted for own use.
- Presentation: Blinding in Clinical Trials
Size: 201,888 bytes, Format: .pptx
This presentation provides more information about the concept of blinding in clinical trials.
- Presentation: Options for Data Collection and Patient-reported Outcomes (PROs)
Size: 611,418 bytes, Format: .pptx
A presentation covering the various types of data collection in clinical trials.
- Presentation: Ethical and Practical Challenges of Organising Clinical Trials in Small Populations.
Size: 495,286 bytes, Format: .pptx
A presentation detailing the ethical and practical challenges of organising clinical trials in small populations.
- Presentation: Paediatric Medicine: The Paediatric Investigation Plan
Size: 479,909 bytes, Format: .pptx
A presentation describing the Paediatric Plan, which can be adapted for own use.
- Presentation: Patient-Reported Outcomes (PROs) Assessment
Size: 408,285 bytes, Format: .pptx
A presentation describing the Patient-Reported Outcomes (PROs) Assessment process, which can be adapted for own use.
- Presentation: Biomarkers
Size: 393,107 bytes, Format: .pptx
A presentation describing biomarkers, which can be adapted for own use.
Additional Learning Resources
Are you ready to elevate your expertise with certifications in areas like Clinical Development, Medicine Discovery, and HTA Evaluation?
Explore the links below to access the EUPATI Open Classroom, choose the courses that match your interests, and embark on your learning adventure today!
- Learn more about the Steps of Making a Medicine!
- Explore Clinical Trials and delve in the Rights & Obligations of trial participants!
- What about Clinical Trials involving Special Populations? Don't Miss Out!
- Grasp key concepts of Statistical Methods used in Clinical Research!
- Deepen Your Understanding of Data Collection!
- Uncover the Practical Application and Vital Role of Patient-Reported Outcomes (PROs).
- Discover the Fundamentals of Early Clinical Development![/glossary_exclude]
Videos
An introduction to clinical research [ECRAN] can be downloaded from EUPATI on YouTube.
Explore the history of clinical trials stemming back to 1747 and learn more about how they work today in this short video from the ECRAN project.
“Clinical Research” by ECRAN Project is licensed under CC BY-NC-SA 4.0
Terms of use - Creative Commons
Remember that all educational content provided by EUPATI is released under a Creative Commons License, which also applies to all derivatives of it!
You can read more about the use of EUPATI content on the Creative Commons page.
Use of the EUPATI logo
The EUPATI logo is protected by trademark and owned by the European Patients Forum.
Except for the limited purpose of indicating that work is created or licensed by EUPATI (European Patients Academy for Therapeutic Innovation), or collaboration with EUPATI, the European Patients Forum (EPF) does not authorise the use, by any party, of the trademark "EUPATI" or any related trademark or logo of EUPATI without the prior written consent of EPF. Any permitted use will be in compliance with EUPATI's then-current trademark usage guidelines, as may be published on its website or otherwise made available upon request from time to time.
A2-SK-protocol-design-V1.0