Last update: 23 November 2015
Introduction
There are several types of clinical trial design. These can be classified as follows:
- according to the method used to allocate participants into treatment or control groups (non-randomised or randomised controlled trials)
- according to the awareness of either participants or researchers or both of which group participants are allocated into (single or double-blind studies)
- according to the magnitude of difference between treatment and control groups that is expected (superiority or non-inferiority trials)
Non-randomised controlled clinical trial designs
In non-randomised controlled trials, participants are allocated into treatment and control arms by the investigator. In these trials, control groups can be concurrent controls or historical controls. When using a historical control, all subjects in the trial receive the study medicine; the results are either compared to the patient’s history (for example, a patient living with a chronic illness) or a previous study control group.
Randomised controlled clinical trial designs
In randomised controlled trials, trial participants are randomly assigned to either treatment or control arms. The process of randomly assigning a trial participant to treatment or control arms is called ‘randomisation’. Different tools can be used to randomise (closed envelopes, computer generated sequences, random numbers). There are two components to randomisation: the generation of a random sequence and the implementation of that random sequence, ideally in a way that keeps participants unaware of the sequence. Randomisation removes potential for bias.
There are different types of randomised trial designs.
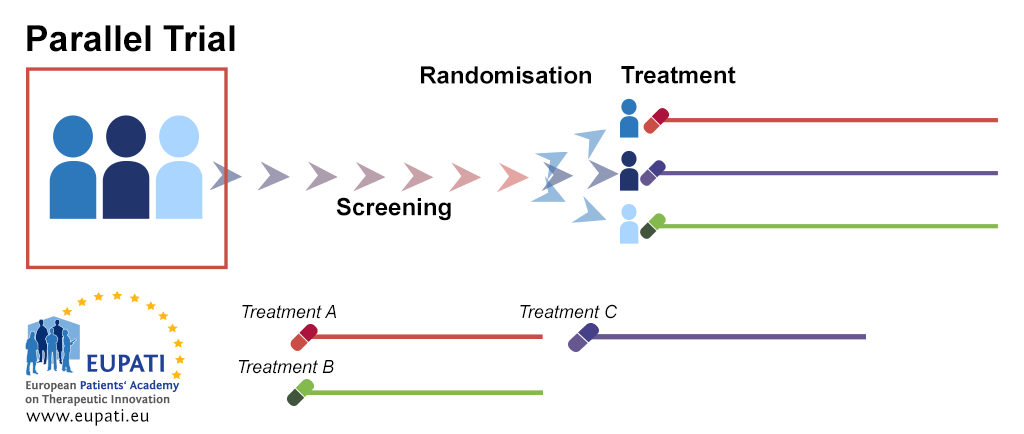
Parallel group trial design
In parallel group randomisation, after randomisation each participant will stay in their assigned treatment arm for the duration of the study. Parallel group design can be applied to many diseases, allows running experiments simultaneously in a number of groups, and groups can be in separate locations.
- After screening, patients are randomised into separate treatment groups. They remain in these treatment arms for the duration of the trial, analysis, and follow-up activities.
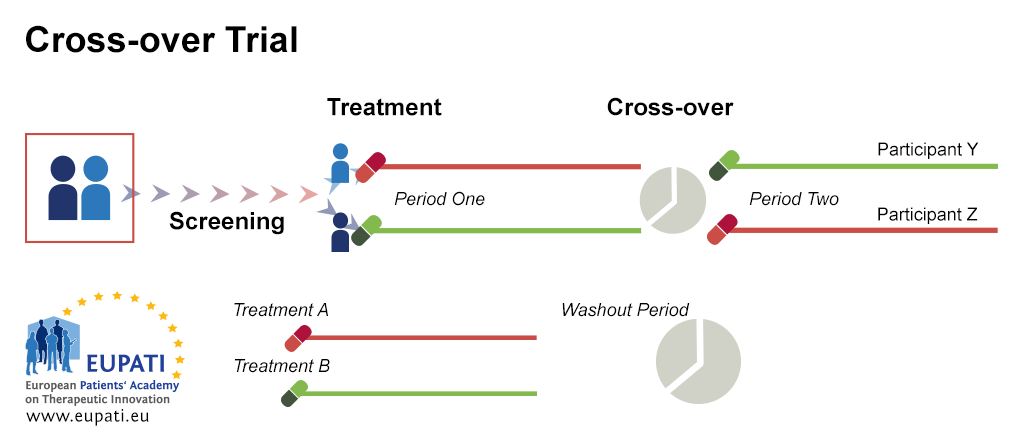
Cross-over trial design
Cross-over randomisation is when participants receive a sequence of different treatments (for example, the candidate compound in the first phase and the comparator/control in the second phase). Each treatment starts at an equivalent point, and each individual serves as his/her own control. It presents some advantages, such as low variance due to treatment and control being the same participant, and the possibility of including a number of treatments. However, there must be a sufficient time gap between the different treatment phases (washout period).
- Patient X and Y are randomised into two different treatment arms. Patient X receives Treatment A during the first period of the study; Patient Y receives Treatment B. After the first period is over, there is a washout period. Patient X then receives Treatment B for the second period of the study while Patient Y receives Treatment A.
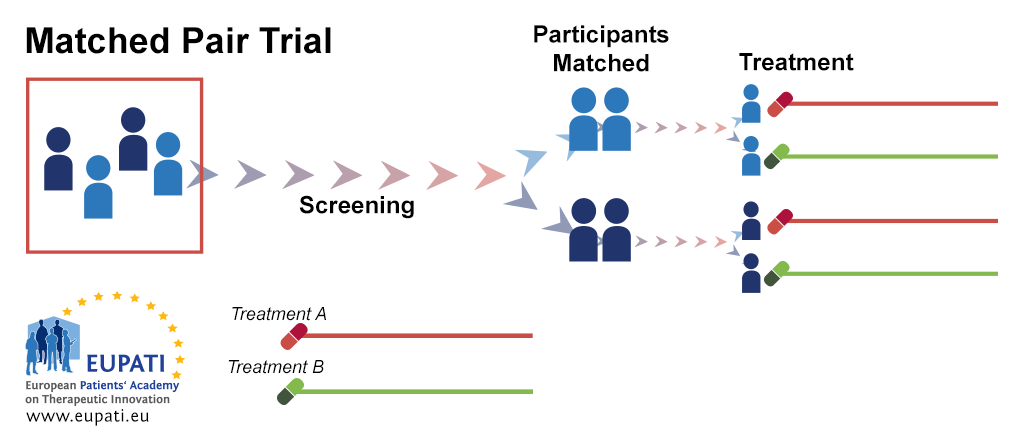
Matched pair trial design
In the matched-pair design, participants are first matched in pairs according to certain characteristics. Then, each member of a pair is randomly assigned to one of the two different study subgroups. This allows comparison between similar study participants who undergo different study procedures.
- After screening, participants are matched into pairs. Within each pair, one participant is randomised onto Treatment A while the other is randomised onto Treatment B.
Stratification
Stratification also allows for comparison between similar study participants who undergo different study procedures. All study participants are grouped according to one or more factors (for example, age, gender, lifestyle factors, concomitant medication) before being randomised. This ensures balanced allocation within each combination.
Cluster sampling
Randomised trials can also use cluster sampling. In cluster sampling, suitable geographical areas are found (for instance, city, region, etc.). A number of these geographical areas are then randomly chosen. For each of these chosen geographical areas, a proportionate subsample from the members of the study sample in that area are chosen, and these subsamples are then combined into a sample group.
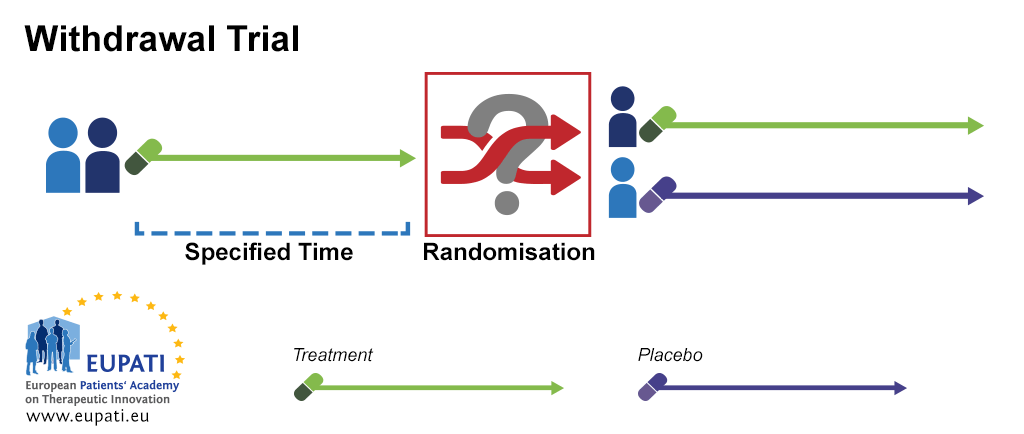
Withdrawal trials
In a withdrawal trial, the participant receives a test treatment for a specified time and are then randomised to continue either with the test treatment or a placebo (withdrawal of active therapy).
- During a withdrawal trial, after the first specified period of time has elapsed, participants are randomised into two groups, one of which receives a placebo instead of continuing to receive the active treatment.
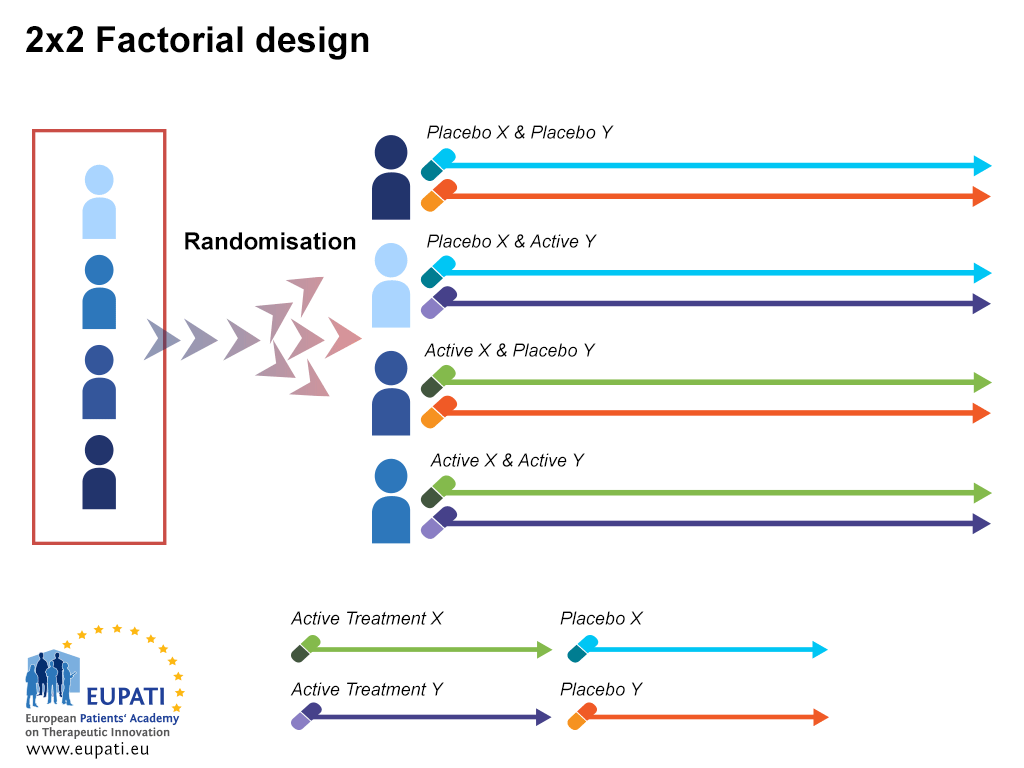
Factorial design
Factorial clinical trials test the effect of more than one treatment. This allows assessment of potential interactions among the treatments.
- An example of a trial using a 2×2 factorial design.
Comparison clinical trial designs
There are a number of different types of comparison trials possible:
- Superiority to demonstrate that the investigational medicine is better than the control;
- Equivalence to demonstrate that the endpoint measure is similar (no worse, no better) than the control;
- Non-inferiority to demonstrate that the investigational medicine is not worse than the control;
- Dose-response relationship trials to determine various dose parameters, including starting dose and maximum dose.
Attachments
- Presentation: Clinical Trial Designs
Size: 1,179,726 bytes, Format: .pptx
A presentation covering the various types of clinical trial design. Details are given about blinding, control, comparisons, and randomisation.
A2-4.30-v1.4