Last update: 3 August 2015
Introduction
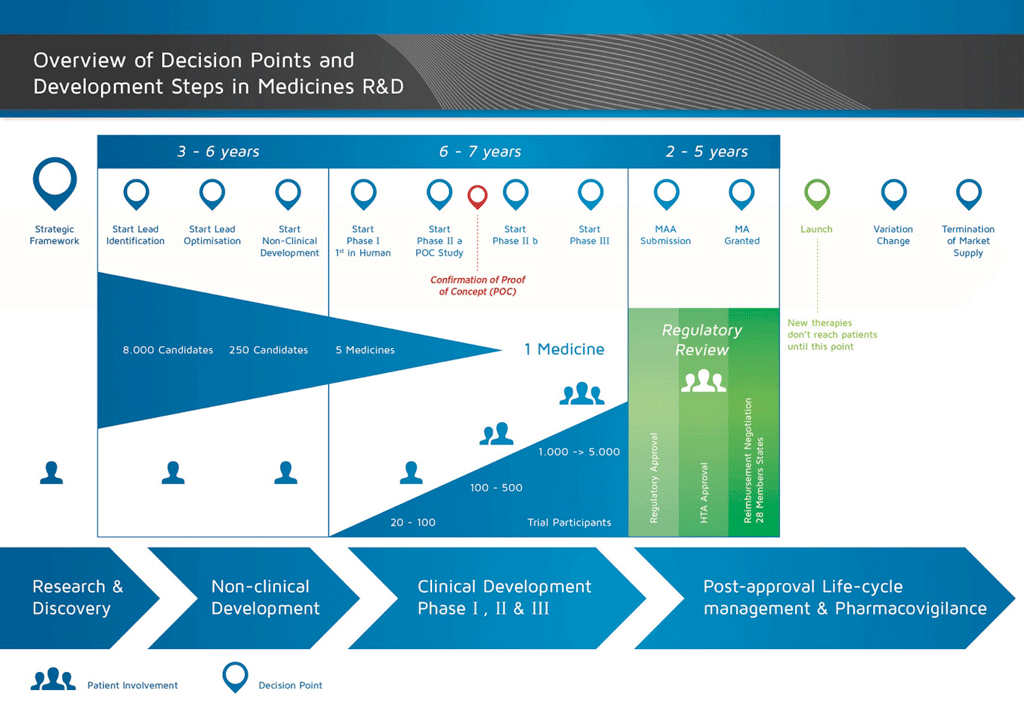
It takes over 12 years and on average costs over €1 billion to do all the research and development necessary before a new medicine is available for patients to use.
Medicines development is a high-risk venture. The majority of substances (around 98%) being developed do not make it to the market as new medicines. This is mostly because when you look at the benefits and risks (negative side effects) found during development do not compare well with medicines that are already available to patients.
The development of a new medicine can be divided into 10 different steps. The following article covers Step 8: Confirmatory studies.
- It takes well over 10 years of careful planning and research for a medicine to go from molecule to a marketable treatment.
Step 8: Development for Launch: Phase III clinical studies – Confirmatory studies
Phase III trials (also called confirmatory studies) are the largest, most complicated, and most expensive part of the development of a medicine. They aim to confirm the efficacy and safety of the candidate compound in a large patient population.
The decision to go on to Phase III trials is only made if it is supported by all the information from earlier studies, as well as manufacturing and business units. The design of such trials is also critically important. Therefore, before Phase III trials begin, there is a large amount of discussion with the external experts, Regulatory Authorities, patient groups, and others. This ensures that the right questions are being asked and the right information is being collected. The number of patients in the trial is also very important so that the results can be properly interpreted and will meet the requirements of the Regulatory Authorities.
All of the information gathered from the earlier stages is used to make important decisions including:
- the final formulation of the medicine (how the active medicine is combined with other chemical substances)
- the dose to be tested
- which patients can be recruited (inclusion criteria)
- which patients cannot be included (exclusion criteria)
- how many patients are required
- the study design
- length of the study
- how efficacy and safety will be measured
- the statistical tests that will be used
At this stage the trials may involve thousands of patients. However, this depends on what the medicine is intended to treat (the ‘indication’). For instance, Phase III trials may be run in smaller populations if the indication is an uncommon condition.
Phase III studies can involve thousands of patients, are run in many countries, and require a huge amount of expertise in order to be effectively run. They are therefore very expensive and time consuming. However, this is the only way to produce a clear picture of the relationship between the efficacy of the medicine (if it works) and its safety (if it is well tolerated), which is very important.
Phase III studies make up the largest, most complicated and most expensive part of the medicine development process. Over 50% of medicines fail in this step. Overall, the failure rate of projects that begin at the discovery stage is more than 97%. The revenue of the few medicines that make it to the market cover the costs of all the projects, the failures as well as the successes.
References
- Edwards, L., Fox, A., & Stonier, P. (Eds.). (2010). Principles and practice of pharmaceutical medicine (3rd ed.). Oxford: Wiley-Blackwell.
Attachments
- Fact sheet: Confirmatory studies
Size: 104,027 bytes, Format: .docx
This fact sheet covers Phase III clinical studies, which are the longest, most complex, and most expensive studies in the medicines development process.
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
A2-1.02.7-v1.1