Last update: 3 August 2015
Introduction
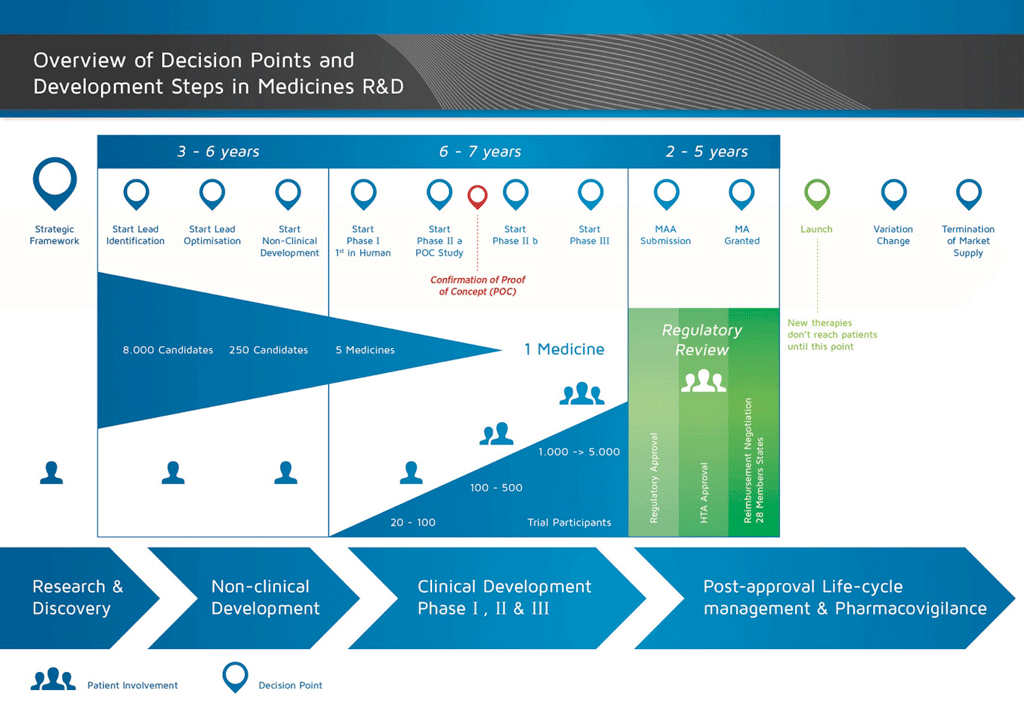
It takes over 12 years and on average costs over €1 billion to do all the research and development necessary before a new medicine is available for patients to use.
Medicines development is a high-risk venture. The majority of substances (around 98%) being developed do not make it to the market as new medicines. This is mostly because when you look at the benefits and risks (negative side effects) found during development do not compare well with medicines that are already available to patients.
The development of a new medicine can be divided into 10 different steps. The following article covers Step 5: Non-clinical safety testing.
- It takes well over 10 years of careful planning and research for a medicine to go from molecule to a marketable treatment.
Step 5: Non-clinical safety testing
Is it safe to proceed to clinical testing? This stage in the medicines development process involves safety testing in animals, which is governed by specific rules and regulations of Good Laboratory Practice (GLP). No candidate medicine can be tested in humans (in clinical studies) before its safety profile has been established in animal safety studies. Medicines development is tightly controlled. The law imposes rules and regulations about what is done, and how it is done.
Before the non-clinical testing work can be carried out, larger amounts of the candidate compound need to be produced so that all the appropriate tests can be completed. This manufacturing process must also follow strict guidelines and regulations, called Good Manufacturing Practice (GMP).
These regulations state which studies must be done and which type of animals must be used to obtain reasonable information. These include looking at effects:
- in the animal overall
- in all the animal’s tissues and organs (systemic toxicology studies)
- on the ability of the animals to reproduce and develop normally (reproductive toxicology studies)
- on the skin or eyes (local toxicology studies)
- any allergies (hypersensitivity studies)
- on the chromosomes and genes (genotoxicity studies)
- any effects on cancer generation (carcinogenicity studies)
These studies are shown below.
Types of toxicology studies
- Systematic toxicology studies
- Reproductive toxicology studies
- Male fertility studies
- Female reproduction and Development studies
- Local toxicology studies
- Hyper sensitivity studies
- Genotoxicity studies
- Carcinogenicity studies
These studies not only show the safety profile in animals, but also provide important information about:
- how the substance enters the body (Absorption)
- Distribution of the substance around the body
- breakdown of the substance by the body (Metabolism)
- how the substance leaves the body (Excretion).
This is sometimes abbreviated to ‘ADME’.
All of this information is used to decide if the candidate compound can proceed into the first human (clinical) study and, if so, what doses to use.
In order to continue into clinical testing with humans, the candidate compound must have shown an acceptable safety profile in all the necessary non-clinical toxicology studies. However, not all of the non-clinical safety studies will have been completed. For example, long-term carcinogenicity studies take on average two years and continue at the same time as the clinical trials.
References
- Edwards, L., Fox, A., & Stonier, P. (Eds.). (2010). Principles and practice of pharmaceutical medicine (3rd ed.). Oxford, UK: Wiley-Blackwell.
Attachments
- Fact Sheet: Medicine discovery
Size: 905,797 bytes, Format: .docx
Medicine discovery. This fact sheet covers the steps of the medicines discovery and development process that occur before a compound can be tested in humans – from pre-discovery (gathering information on a disease) to non-clinical safety testing in animals.
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
A2-1.02.4-v1.1