Last update: 3 August 2015
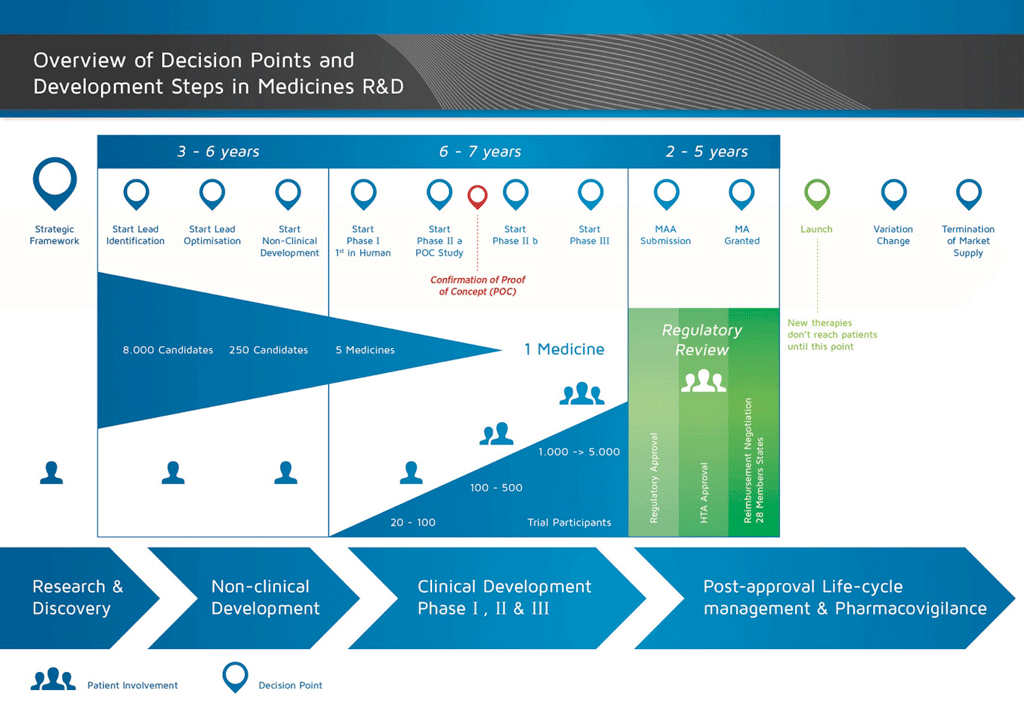
It takes over 12 years and on average costs over €1 billion to do all the research and development necessary before a new medicine is available for patients to use.
Medicines development is a high risk venture. The majority of substances (around 98%) being developed do not make it to the market as new medicines. This is mostly because the benefits and risks (negative side effects) found during development do not compare well with medicines that are already available to patients.
The development of a new medicine can be divided into 10 different steps. The following article covers Step 1: Pre-discovery.
- It takes well over 10 years of careful planning and research for a medicine to go from molecule to a marketable treatment.
Step 1: Pre-discovery
Determining if there is an ‘unmet need’. In the pre-discovery phase, scientists in academia (universities) and in the industry (pharmaceutical companies) work to understand the disease.
An unmet need refers to a disease where either:
- there is no suitable medicine available, or
- there is a medicine but some patients might have unacceptable side effects and are not able to take it.
The research and development process uses lots of resources and is expensive. Companies may only start a new programme for an unmet need if there is a commercial case to do so. This is because companies need to profit from the new medicine in order to pay for the costs of development and to invest in projects for new medicines. There are many unmet needs for which new medicines are not currently being developed. European legislators are aware of this, and they offer incentives and rewards to support the development of medicines in more difficult cases, for example for children or patients with rare diseases.
The basic steps in medicines development are shown in the diagram. One important step is the regulatory submission and approval process which must be successfully completed before the medicine can be marketed (launched). However, the success of this approval process is not in the hands of the company.
Each step in the medicines development process involves getting an agreement for the money (investment) and the people to do the work – called an ‘investment decision’ (ID). The results of each step are then reviewed before the next activity can begin. This pattern of investment decision – activity – results – investment decision continues for the entire development process. This means that if the results at any stage are not satisfactory, the project will be stopped. The financial and human resources can then be directed to other projects.
References
- Edwards, L., Fox, A., & Stonier, P. (Eds.). (2010). Principles and practice of pharmaceutical medicine (3rd ed.). Oxford: Wiley-Blackwell.
Attachments
- Fact Sheet: Medicine discovery
Size: 905,797 bytes, Format: .docx
Medicine discovery. This fact sheet covers the steps of the medicines discovery and development process that occur before a compound can be tested in humans – from pre-discovery (gathering information on a disease) to non-clinical safety testing in animals.
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
A2-1.02.1-V1.1