Last update: 3 August 2015
Introduction
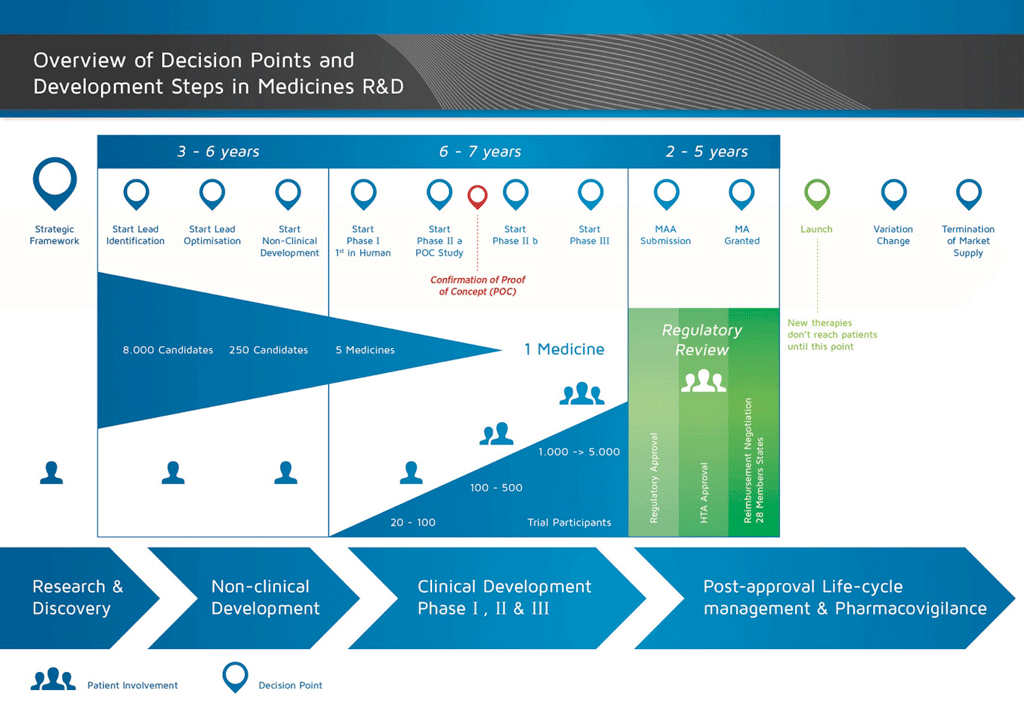
It takes over 12 years and on average costs over €1 billion to do all the research and development necessary before a new medicine is available for patients to use.
Medicines development is a high-risk venture. The majority of substances (around 98%) being developed do not make it to the market as new medicines. This is mostly because when you look at the benefits and risks (negative side effects) found during development do not compare well with medicines that are already available to patients.
The development of a new medicine can be divided into 10 different steps. The following article covers Step 10: Marketing and post-marketing safety surveillance and life-cycle management.
- It takes well over 10 years of careful planning and research for a medicine to go from molecule to a marketable treatment.
Marketing and post-marketing safety surveillance
The marketing process involves sharing the information about the new medicine with doctors and other health care professionals so that they become aware of the effects of the new medicine, and may prescribe it in cases where they believe patients can benefit.
However, the development process does not stop there. There is still a need to collect and analyse the information about the safety of the medicine when it is used in ‘real life’ (called ‘pharmacovigilance’). This is because:
- In clinical trials (which are designed to give clear answers), patients ideally only have the illness being studied and are not taking any other medicines.
- In real life, a large number of patients take the new medicine. They may have several other illnesses and take a variety of other medicines.
Data from both clinical trials and real life are necessary to fully understand the real risk-benefit relationship of the medicine.
Life-cycle management
After a medicine enters the market, the development process continues to explore:
- other possible uses (indications) for the medicine. For example, if the initial use was for patients with asthma, a new indication might be for patients with a different type of lung disease, for example a chronic obstructive pulmonary disease.
- improved ways of making and using the medicine (new formulations). For example, a special formulation for children.
All of these activities are known as ‘life-cycle management’.
Other changes in the life cycle of a medicine
When a medicine is first marketed, it is protected by a ‘patent’. This means that other companies cannot market a similar medicine. At the end of the patent or data protection period other companies will manufacture and market the same product. When this happens, the product is called a ‘generic’.
New medicines are usually licensed as Prescription-Only Medicines (POM). This means that healthcare professionals can supervise their use in the first few years. Where it is appropriate and safe to do so, the medicine can then be made available as an Over-The-Counter (OTC) medicine. This involves a change in the regulatory status of the medicine and a new licence is required. Patients can buy the OTC medicine directly from a pharmacy or supermarket (depending on the country).
References
- Edwards, L., Fox, A., & Stonier, P. (Eds.). (2010). Principles and practice of pharmaceutical medicine (3rd ed.). Oxford: Wiley-Blackwell.
Attachments
- Fact sheet: Safety surveillance and life-cycle management
Size: 104,426 bytes, Format: .docx
Safety surveillance and life-cycle management. This fact sheet covers the activities that occur after a medicine has received authorisation from the regulatory authorities. These include marketing, safety surveillance, and other life-cycle management activities.
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
A2-1.02.9-v1.1