Last update: 3 August 2015
Introduction
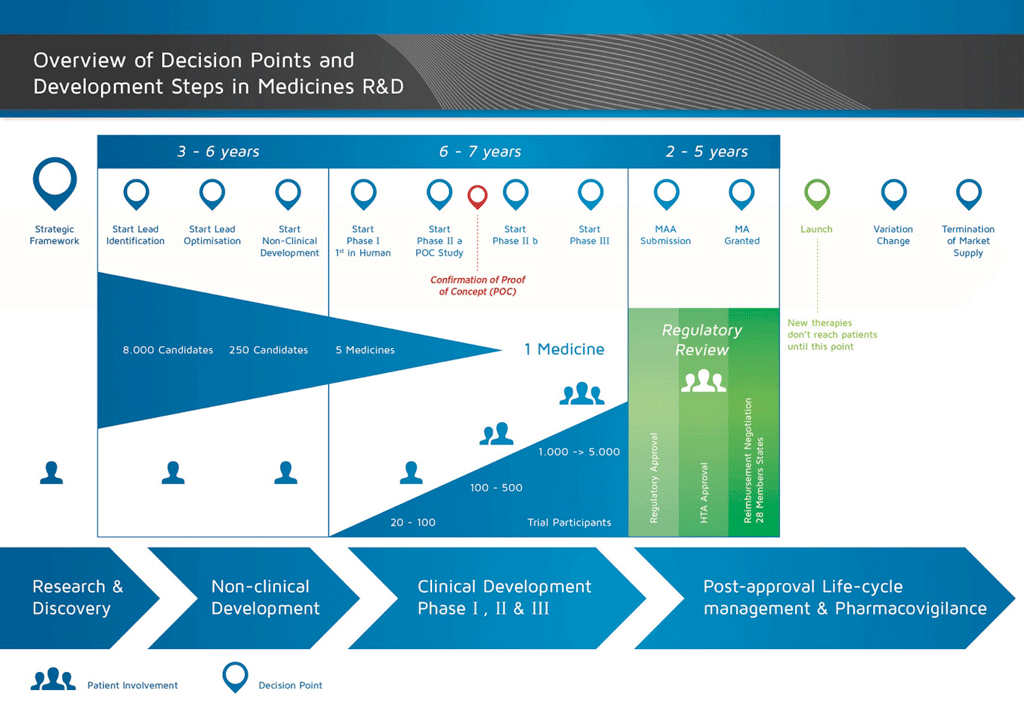
It takes over 12 years and on average costs over €1 billion to do all the research and development necessary before a new medicine is available for patients to use.
Medicines development is a high-risk venture. The majority of substances (around 98%) being developed do not make it to the market as new medicines. This is mostly because when you look at the benefits and risks (negative side effects) found during development do not compare well with medicines that are already available to patients.
The development of a new medicine can be divided into 10 different steps. The following article covers Steps 7: Proof of concept – Phase II clinical studies.
- It takes well over 10 years of careful planning and research for a medicine to go from molecule to a marketable treatment.
Step 7: Proof of concept – Phase II clinical studies
Trials in patients. Once the volunteer study results have shown that it is safe to proceed, the next step is to start clinical trials in patients with the disease that is being treated. The same guidelines and regulations apply to these trials as Phase I trials.
In Phase II and Phase III studies, there are usually two treatment groups. One group has the active medicine and one group receives the current best treatment, or a dummy medicine which has no effect on the body (called a ‘placebo’). These studies are usually run as ‘double-blind’, ‘randomised’ studies.
- ‘Double-blind’ means that both the doctor and the participant do not know who is receiving the active medicine or current best treatment/placebo.
- ‘Randomised’ means that the treatment groups are chosen by chance. This is usually done with a computer that generates a random code. It cannot be influenced by the doctor or anyone else.
- ‘Placebo-controlled’ means that some participants will receive a placebo given under the exact same conditions as the active medicine. This allows the effects related to the medicine to be separated. For example, if a participant in a study complains of a headache it is important to know if that is related to the active medicine. If the same number of participants receiving placebo complain of headaches, this shows that the headache cannot be due only to the active medicine.
All the details of the trial are described in the Study Protocol and the information is collected in the Case Record Form (CRF). The results are then analysed using statistical tests.
These trials are usually carried out in 100 to 500 patients. They are designed to get information about the effect of the medicine on the actual disease (‘proof of concept’). This is also the stage at which different doses of the medicine are used to find out which is the best dose. This dose is then used for the next phase of larger clinical studies.
The more that can be learned about the effect in the patients at this stage, the easier it is to decide if the development of the candidate compound should continue. However, Phase II studies are too small to be able to provide sufficient evidence about efficacy and safety. Therefore, it is important to build up more and more information about how the medicine works in patients in order to reduce the risk of failing at the next stage (Phase III or Development for Launch), which is the most complicated and expensive phase of development
Because these Phase II studies are carried out in patients, the studies are usually run in several hospital sites by hospital doctors – called investigators – in contrast to Phase I studies, which are usually performed in special units.
Conducting trials in several different sites at the same time is more complicated than conducting a trial in a single site:
- All investigators and study nurses must be trained using an established protocol so that the study is conducted in the same way at all the sites.
- The medicine must be exported to different countries and stored properly in different pharmacies.
- Blood samples collected from patients in the clinical trial are usually sent to a single central laboratory.
- All the local country rules and regulations must be understood and followed.
- Ethics Committee opinion and National Competent Authority (NCA) approval is usually required in each country.
All of these activities must be coordinated by the global study team.
Summary: Steps 1-7
By the end of Phase II studies, the programme will have:
- taken an average of 8.5 years, and
- cost an average of €1 billion.
Of every 10 medicines that are tested in Phase I and Phase II, only two (on average) will continue to the next phase.
References
- Edwards, L., Fox, A., & Stonier, P. (Eds.). (2010). Principles and practice of pharmaceutical medicine (3rd ed.). Oxford: Wiley-Blackwell.
Attachments
- Fact Sheet: Proof of Concept
Size: 104,272 bytes, Format: .docx
This fact sheet covers Phase II clinical studies – or proof of mechanism studies. These studies are carried out in small number of patients suffering from the target disease in order to ascertain that the candidate compound effects the disease.
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
A2-1.02.6-v1.1