Last update: 13 January 2016
Introduction
The normal development of a medicine requires that various studies be performed to ensure its quality, safety, and efficacy. These studies, in turn, require careful planning procedures so that they are sure to be ethically and scientifically valid. During the development process, a Paediatric Investigation Plan is written to ensure that the necessary data on the use of the medicine in children are obtained when it is safe to do so.
Paediatric Investigation Plan (PIP)
The aim of a PIP is to support the medicine’s authorisation in children. Once the PIP is agreed upon, it is kept up-to-date through a modification procedure.
A PIP will contain the following:
- The needs of all age groups of children, from birth to adolescence, and the timing of studies in children compared to adults. The full paediatric age range should be covered unless justified.
- An overview of the disease, its diagnosis and treatment. It should highlight any differences between children and adults.
- An overview of the data available for the medicine, including:
- Chemical information on the current formulation
- Non-clinical and clinical study data
- Proposed strategy including some or all of the following:
- A description of any additional non-clinical studies
- Plans for a paediatric formulation (if required), including measures to adapt the medicine’s formulation to make its use more acceptable in children, such as use of a liquid formulation rather than large tablets
- A description of planned clinical trials or modelling/simulation (using computers to predict how the medicine may act in children), including:
- Details of the timing of studies in children compared to the adult development plan
- If the studies in children will not be completed in parallel, a deferral may be requested for the completion of the PIP.
The PIP is submitted by the applicant to the Paediatric Committee (PDCO). Legislation requires the submission of a PIP ‘no later than the completion of adult human pharmacokinetic studies’. Usually, the PIP is submitted once there is adult clinical experience. If a PIP is completed and paediatric data is included in the EU product information, a six month extension to the Supplementary Protection Certificate (SPC) is granted as a reward.
The EMA Paediatric Committee (PDCO)
The Paediatric Committee (PDCO) assesses the content of a PIP, any requests for waivers and deferrals, and provides expert analysis and a critical view in order to adopt an ‘opinion’ to determine whether or not the PIP is acceptable. The PDCO includes patient representatives.
On request, the Committee will assess data generated after a PIP has been agreed upon. They also maintain:
- A list of medicine classes or products for waivers
- An updated inventory of established paediatric needs through collected data.
They use this information to advise European paediatric research networks on diseases that need to be studied and where paediatric formulations of existing medicines are needed.
The EU legislation is continuously reviewed and revised, which provides an opportunity to optimise the process of getting medicines to patients. Input is requested from all stakeholders, including doctors, healthcare professionals, pharmaceutical companies, patients, and patient organisations.
Overview of the PIP procedure
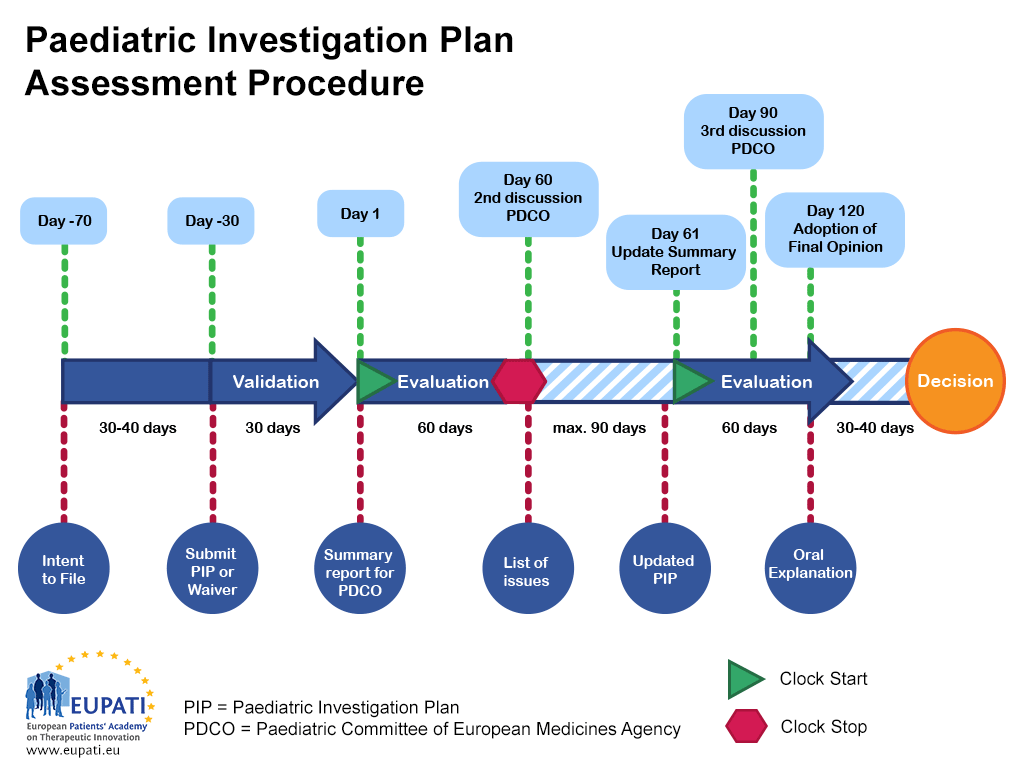
- A Paediatric Investigation Plan is assessed by the Paediatric Committee of the European Medicines Agency and follows a set procedure with defined timelines.
The PIP procedure takes 9 to 10 months from submission to decision. Once the applicant submits an ‘intent to file’, the PDCO will appoint a ‘rapporteur’ to lead the assessment and a ‘peer reviewer’ to check the quality of the assessment from within the Committee. The rapporteur and peer reviewer check the initial PIP and present their findings to the PDCO.
The review process takes the form of a 120-day procedure; however, there is a pause (clock stop) at Day 60 that allows the PDCO to ask questions of the applicant. These clock stops are usually a maximum of three months long, although the length is agreed upon with the PDCO on a case-by-case basis.
Once the applicant’s responses have been submitted, the clock begins again at Day 61 and the procedure runs again without pause until it finishes at Day 120. This means that any outstanding questions must be addressed during the procedure. If any questions remain outstanding after the third discussion with the PDCO, then the PDCO or the applicant can request an oral explanation. This enables the applicant to speak directly to the whole Committee.
PIP waivers
A waiver exempting the sponsor from submitting a PIP may be granted when:
- The medicine is likely to be ineffective or unsafe in children;
- The disease or condition occurs only in adults; or
- There is a lack of ‘significant therapeutic benefit’ observed, or there is a justification that there are feasibility issues meaning that significant therapeutic benefit cannot be demonstrated.
There are three types of waiver:
- Class waiver – according to a list issued by the PDCO of conditions that only occur in adults. The PDCO currently considers the removal of all class waivers (mostly related to cancers)
- Full waiver – for all paediatric subset(s) and indication(s)
- Partial waiver – for one or more paediatric subset(s) and indication(s)
A waiver may be reviewed and changed. If a waiver is revoked, the requirement to submit data according to an agreed PIP will not apply for 36 months.
Further Resources
- For further information on PDCO roles, see: European Medicines Agency (2010). Roles and responsibilities of members and alternates, rapporteur and peer reviewers, experts and observers of the Paediatric Committee (PDCO). Retrieved 25 August, 2015 from https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/role-and-responsibilities-members-and-alternates-rapporteur-and-peer-reviewers-experts-and-observers-paediatric-committee_en.pdf
- For further information on the PDCO, see: European Medicines Agency (2015). PDCO: Overview. Retrieved 5 July, 2021 from https://www.ema.europa.eu/en/committees/paediatric-committee-pdco
- For frequently asked questions on the PIP procedure, see: European Medicines Agency (2014). Questions and answers on the procedure of PIP compliance verification at EMA, and on paediatric rewards. Retrieved 25 August, 2015 from https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/questions-and-answers-procedure-paediatric-investigation-plan-compliance-verification-european-medicines-agency-and-paediatric-rewards_en.pdf
Attachments
- Presentation: Paediatric Medicine: The Paediatric Investigation Plan
Size: 479,909 bytes, Format: .pptx
A presentation describing the Paediatric Plan, which can be adapted for own use.
A2-1.18.4-v1.1
- Presentation: Paediatric Medicine: The Paediatric Investigation Plan