Last update: 26 September 2016
What is Early Clinical Development?
Early Clinical Development refers generally to the first studies of a medicine in humans – typically known as Phase I and Phase II trials.
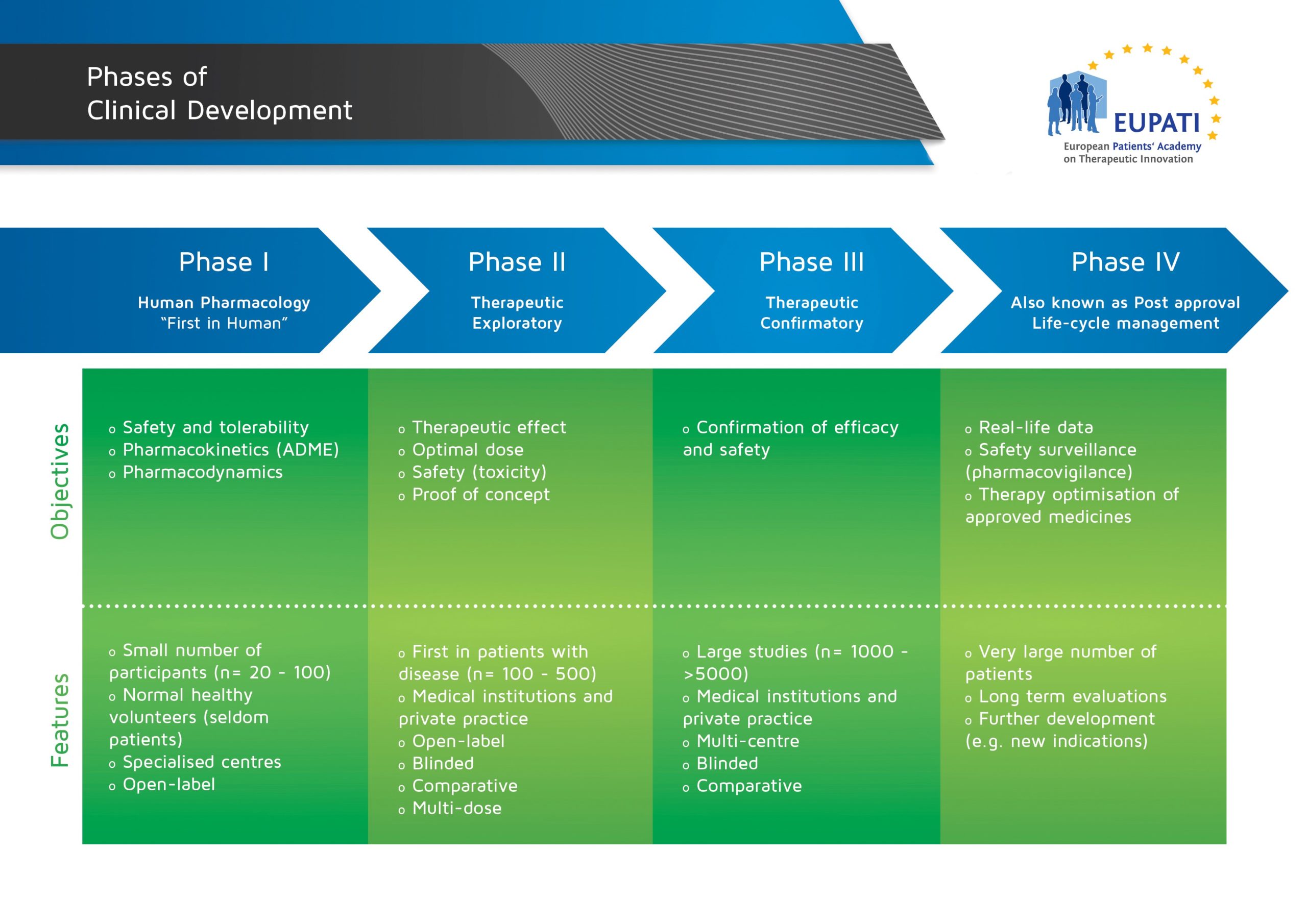
The stages of clinical development are usually represented as consecutive phases, as shown in the image below:
- The four phases of clinical development differ in terms of their objectives and features.
The logic behind representing medicines development in a series of consecutive phases comes from the idea that the results of prior studies should influence the plans for later studies: emerging data will frequently initiate modifications of the development strategies.
However, studies are also classified into phases based not just on when they occur, but also on their objectives. In some cases, studies may combine several phases with different fundamental objectives.
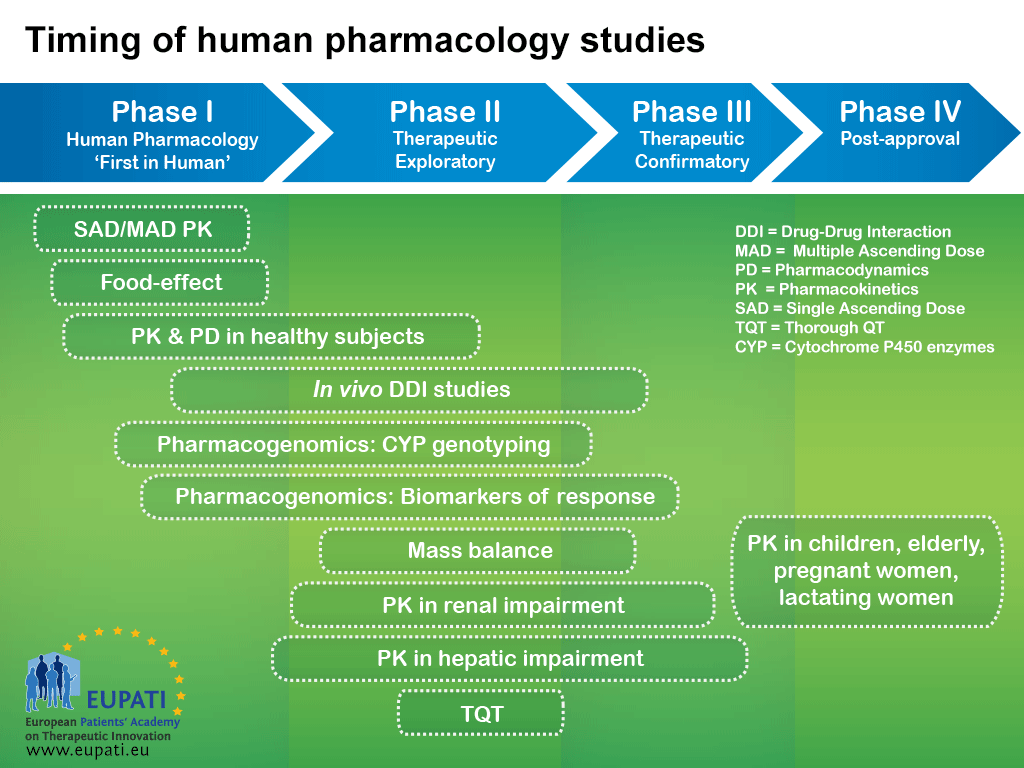
This means that as the development of a medicine progresses, new data may suggest the need for additional studies that are typically part of an earlier phase. For example, although human pharmacology studies are typically conducted during Phase I, many of these studies are also conducted during each of the later phases (see diagram below).
- The logic behind representing medicines development in a series of consecutive phases comes from the idea that the results of prior studies should influence the plans for later studies: emerging data will frequently initiate modifications of the development strategies.
What are the objectives of Early Clinical Development?
Studies in early clinical development focus on the safety and tolerability of the new medicine. They also try to show that the medicine can have the intended effect.
The following key questions must be answered during early clinical development:
-
Phase I
- Is the medicine safe in humans? At what levels? (Tolerance)
- What does the body do to the medicine? (Pharmacokinetics (PK))
- What does the medicine do to the body? (Pharmacodynamics (PD))
- Is the medicine active?
-
Phase II
- Is the medicine safe in patients? (Safety)
- What does the medicine do to the body? (Pharmacodynamics (PD))
- Does the medicine seem to work in patients? At what dose(s)? (Effect)
- How should confirmatory trials be designed? (Endpoints, target population, other medications being taken (concomitant), etc.)
- What interactions are there? (Drug-Drug interactions, interactions with food and drink, etc.)
What are the requirements for early clinical development?
Before early clinical development of a medicine can begin, there must be enough data from non-clinical studies supporting the medicine’s safety for human administration. Then, a clinical development plan must be compiled which:
- Establishes the objectives of the clinical programme.
- Sets out the requirements that must be fulfilled in order to consider a Proof of Concept as positive.
- Describes the design and conduct of Phase I and Phase II clinical studies.
How are decisions made during early clinical development?
Development decisions are data-driven. The results of studies are carefully considered before development continues. The Proof of Concept must be achieved and a dosage schedule selected before further development can continue. If early clinical development of a medicine returns positive results, then more testing of the medicine can continue. Unclear results during early clinical development require further testing and assessment before decisions can be made. If early clinical development of a medicine returns negative results – for instance, if the concept is not adequately proven or unacceptable safety issues arise – then development of the medicine stops. This is the point at which the development of a large number of potential medicines must be discontinued.
A2-5.03.1-V1.2