Last update: 4 August 2015
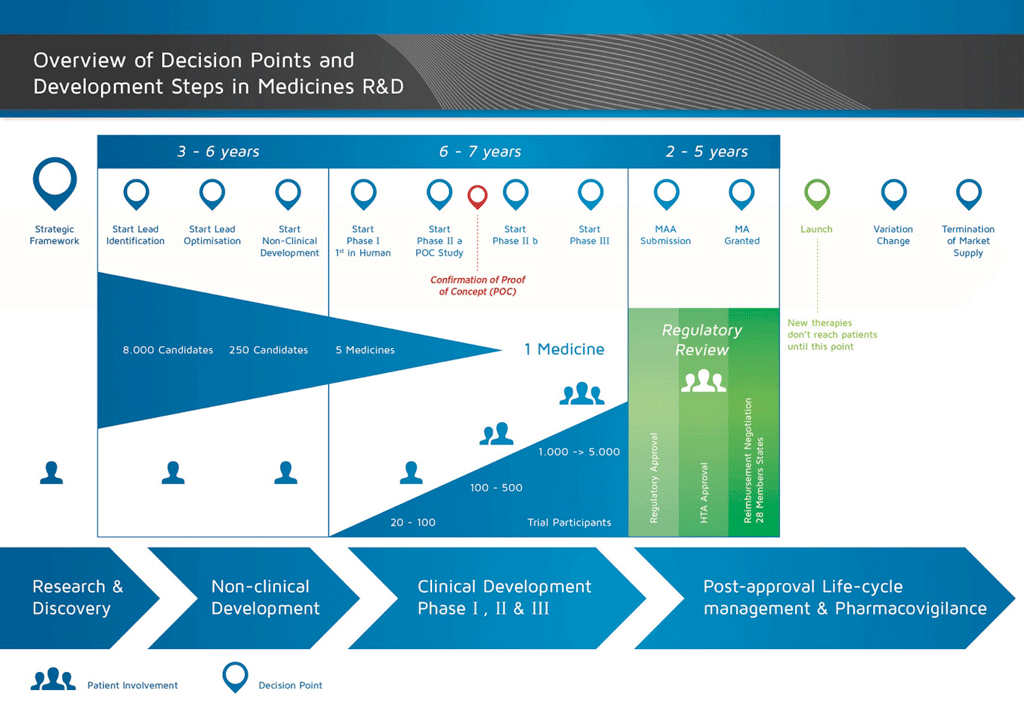
It takes over 12 years and on average costs over €1 billion to do all the research and development necessary before a new medicine is available for patients to use.
Medicines development is a high-risk venture. The majority of substances (around 98%) being developed do not make it to the market as new medicines. This is mostly because when you look at the benefits and risks (negative side effects) found during development do not compare well with medicines that are already available to patients.
The development of a new medicine can be divided into 10 different steps. The following article covers Step 2: Target Selection.
- It takes well over 10 years of careful planning and research for a medicine to go from molecule to a marketable treatment.
What is a 'target'?
Diseases occur when the normal body processes are altered or not functioning properly. When developing a medicine, it is important to understand in detail (at the level of the molecules) what has gone wrong. This means that the abnormal process can then be ‘targeted’ and corrected. The ‘target’ may be: a molecule that has been produced in excess, interfering with normal body function; a molecule that is not being produced in normal amounts; or a molecule that has an abnormal structure. For example, in diabetes, there is either a lack of insulin production or cells don’t respond to it, and in cancer there can be too much of a chemical messenger signalling the cells to grow abnormally.
How does 'targeting' work?
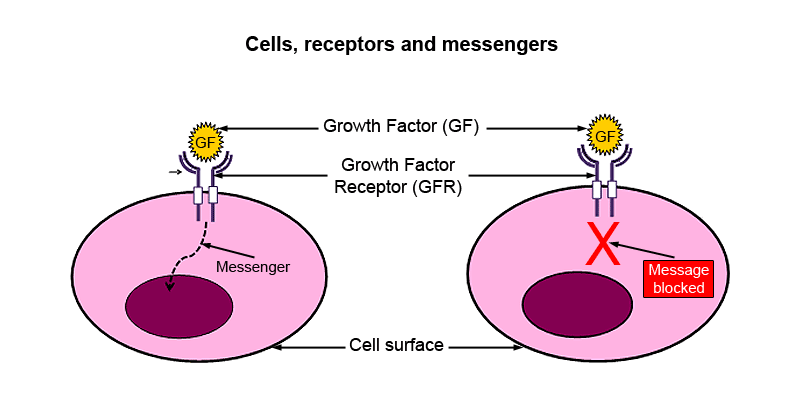
The figure below shows a simple representation of a cell with a nucleus and a receptor on the cell surface.
- The nucleus acts as the control centre for the cell – it contains the genetic material.
- The receptor allows chemical messengers to communicate with the nucleus.
When a chemical messenger, in this case the ‘growth factor’, combines with the growth factor receptor on the cell surface, a message is generated inside the cell. This then communicates with the nucleus, which then stimulates the cell to divide. When the signalling is uncontrolled, the cellular growth leads to cancer. Blocking the receptor in cancer cells will prevent transmission of the message to the nucleus and thus prevent uncontrolled cell growth.
If you can block the receptor in cancer cells, this will:
- stop the message being sent, and
- prevent uncontrolled cell growth.
The ‘target’ in this example is therefore the growth factor receptor.
- Cells, receptors and messengers. The growth factor, a chemical messenger, combines with the growth factor receptor on the surface of the cell, triggering a message to the nucleus. Blocking the receptor will prevent transmission of the message and thus uncontrolled cellular growth. The ‘target’ in this diagram is the growth factor receptor.
The importance of target selection
In many cases it is impossible to know in detail what has gone wrong. Often in a disease, there are several abnormalities, or ‘targets’, but scientists cannot tell precisely which target is responsible for the disease. It could also be that these abnormalities have not caused the illness and therefore trying to correct them will not treat the disease. In this case, the development project might be pursuing the wrong target, and ultimately it will fail. This means that selecting the best target to work on in a project is crucial.
References
- Edwards, L., Fox, A., & Stonier, P. (Eds.). (2010). Principles and practice of pharmaceutical medicine (3rd ed.). Oxford, UK: Wiley-Blackwell.
Attachments
- Fact Sheet: Medicine discovery
Size: 905,797 bytes, Format: .docx
Medicine discovery. This fact sheet covers the steps of the medicines discovery and development process that occur before a compound can be tested in humans – from pre-discovery (gathering information on a disease) to non-clinical safety testing in animals.
- Presentation: The basic principles of medicine discovery and development
Size: 918,164 bytes, Format: .pptx
The basic principles of medicine discovery and development. It takes over 12 years and over €1 billion to do all the research and development necessary before a new medicine is available for patients to use. This presentation details the process from discovery to release of a new medicine onto the market and beyond.
A2-1.02.2-V1.1