Non-clinical testing
« Back to Glossary IndexNon-clinical testing is conducted at a stage of medicines development that uses animals and/or cells or tissues. It does not involve testing in humans. The main goal of non-clinical tests is to determine the safety of a medicine. Non-clinical testing will investigate any harmful effects of the medicine on the body due to the medicine’s pharmacology, such as:
- Toxic effects – for example, on the reproductive system
- If the medicine causes genetic changes
- For some substances, whether they might cause cancerous growth.
Toxicity will be measured in relation to different doses, or length of use of the medicine. The reversibility of any toxicity will also be studied.
Information from non-clinical testing is used in planning clinical trials in humans. It is used to decide what the starting dose should be, and the range of does to be tested. It also suggests what clinical signs should be looked for in order to detect any adverse effects.
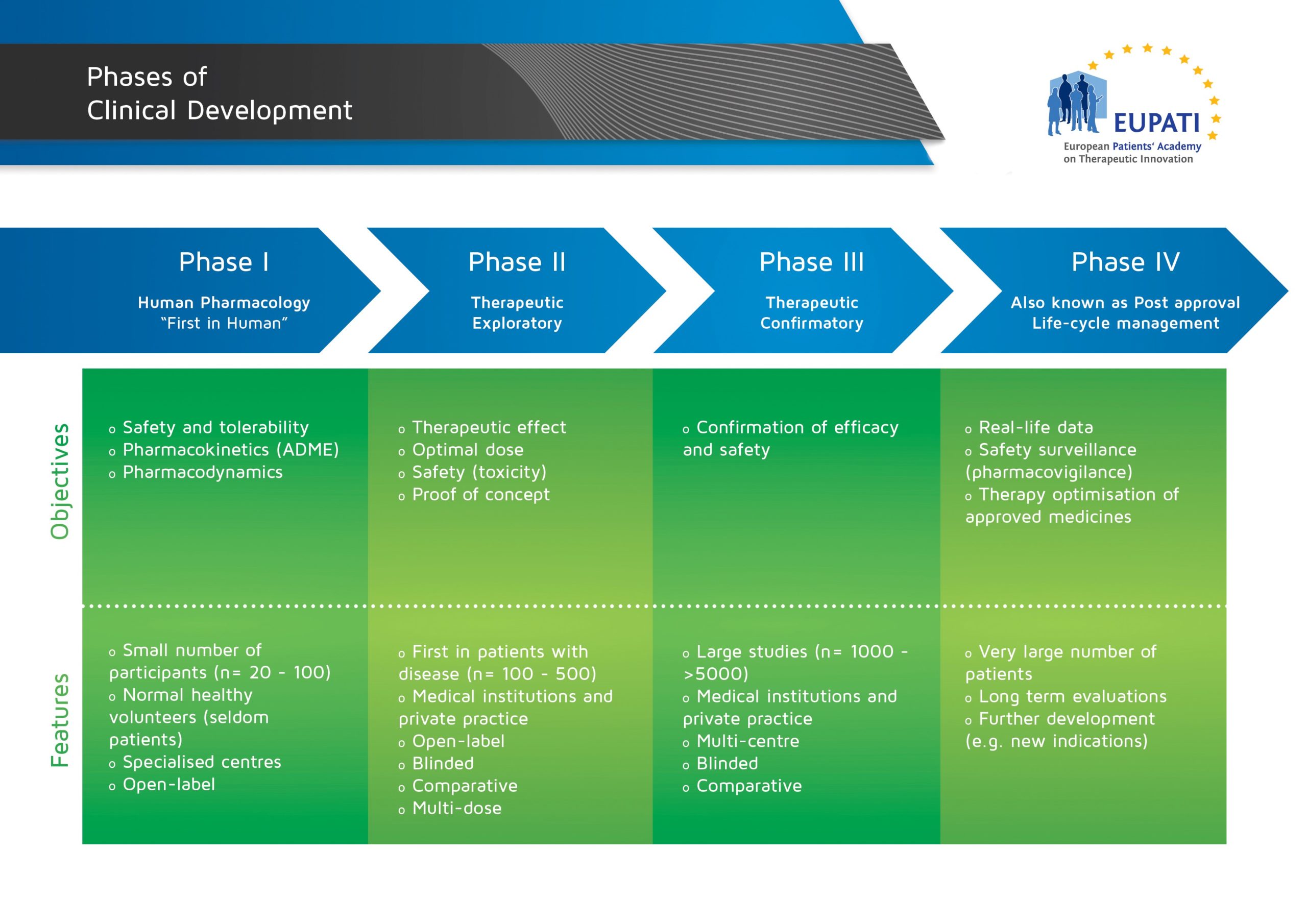
- The four phases of clinical development differ in terms of their objectives and features.