Phase I Trials
« Back to Glossary IndexNormally, the first studies in humans with a new medicine are Phase I trials.
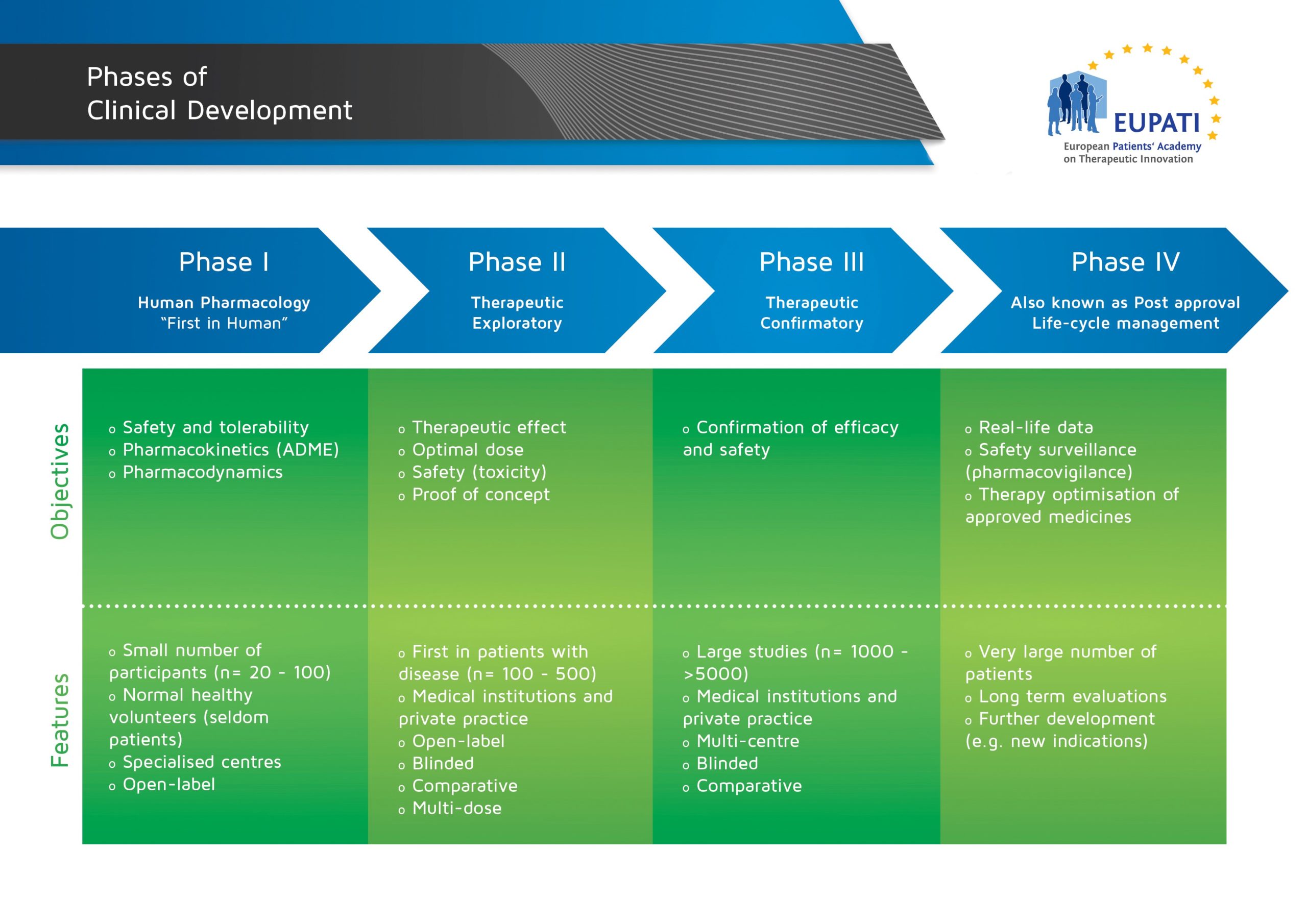
Phase I trials are usually conducted in a small number of healthy volunteers (although some trials recruit patients). The aim of Phase I trials is to find out the safe dose range, and to look for any side effects. The initial dose given will be very small, and gradually increased if no or only mild side effects are observed. A new medicine has to meet certain pre-set requirements before it can continue to Phase II trials. Phase I, II, and III trials are commonly known together as ‘clinical development’.
- The four phases of clinical development differ in terms of their objectives and features.
Synonyms:
Phase I Study, Phase I Studies