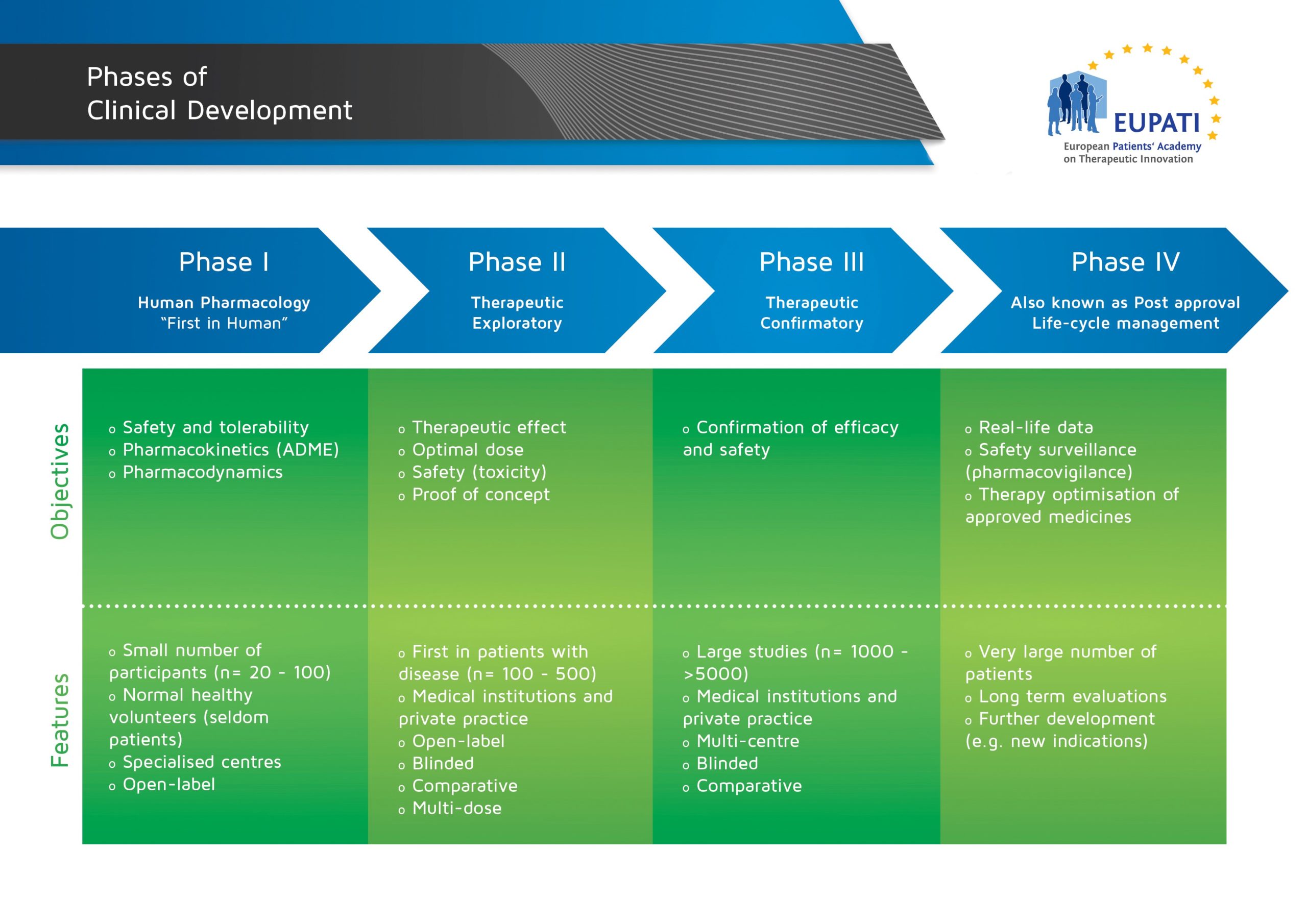
Phase II Trials
« Back to Glossary IndexPhase II trials are generally the first studies with a new medicine in patients. They are usually conducted in a small number of patients who are monitored closely. These trials are often larger than Phase I trials.
Phase II studies are designed to find out if the medicine has a beneficial effect on the disease in question: They might compare the new medicine to an existing treatment or to a placebo. They also set out to determine the best dose range and how often the medicine should be given, and investigate the best way to manage any side effects.
A new medicine has to meet certain pre-set requirements before it can continue to Phase III trials. Phase I, II, and III trials are commonly known as ‘clinical development’.
- The four phases of clinical development differ in terms of their objectives and features.