Phase III Trials
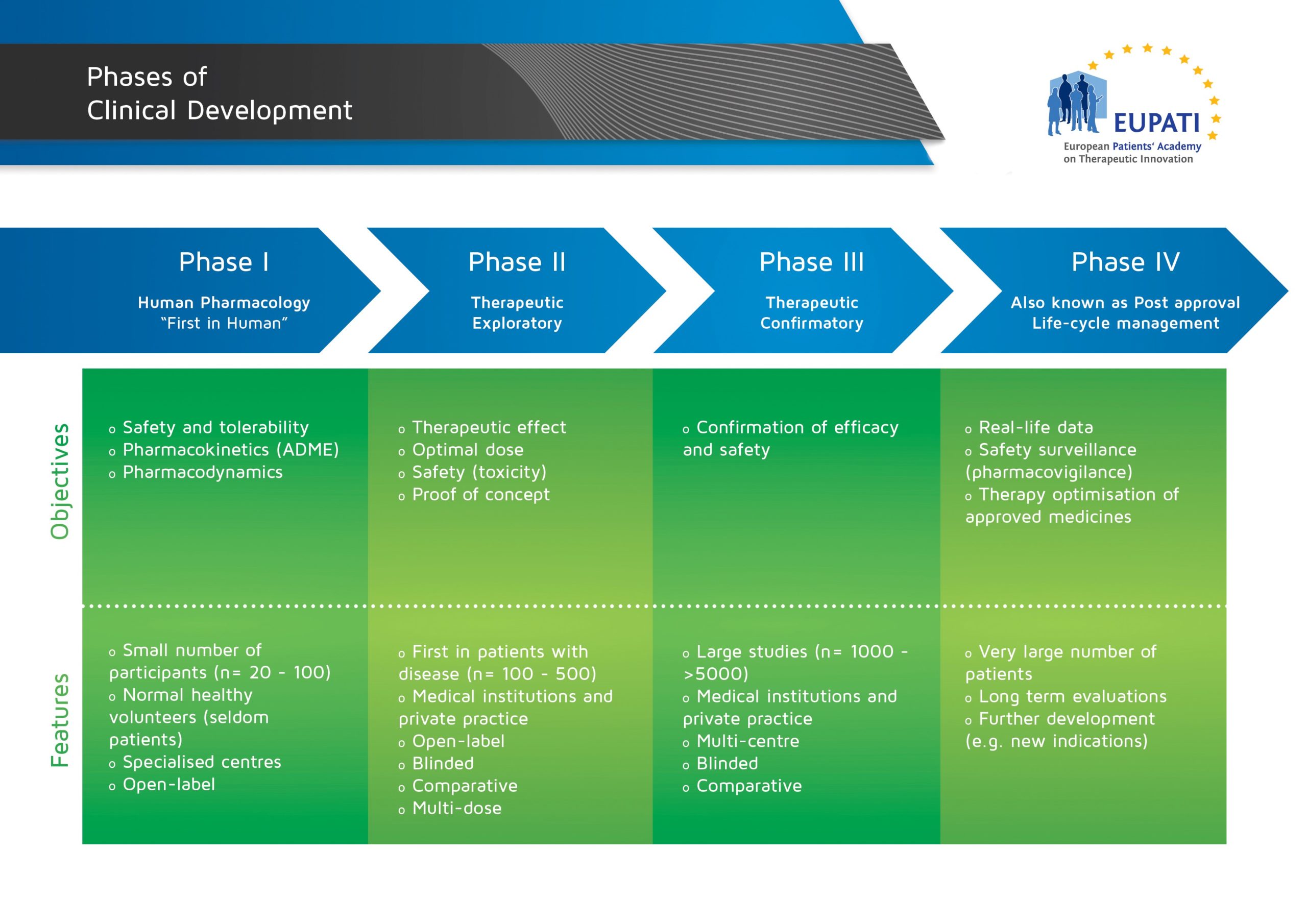
« Back to Glossary IndexPhase III trials are generally large (comprising thousands of patients) and involve several study sites, sometimes in different countries. They compare the new medicine to existing treatments or a placebo, in order to show the safety and efficacy of the new medicine. Most Phase III trials are randomised.
Phase I, II, and III trials are commonly known as ‘clinical development’. Phase III studies are critical to applications for marketing authorisation.
- The four phases of clinical development differ in terms of their objectives and features.
Synonyms:
Phase III Study, Phase III Studies, Confirmatory, Phase 3