Last update: 19 November 2015
Introduction
Toxicity studies investigate the safety profile of the candidate compound. They also provide important information about the absorption, distribution, metabolism, and excretion (ADME) of the compound in the body. A candidate compound must be assessed in many different kinds of non-clinical toxicity study before it can be administered to the first human volunteer; even more toxicity studies are required thereafter before the medicine receives marketing authorisation. The following article explores the various types of toxicology study that may be necessary to include in a non-clinical programme.
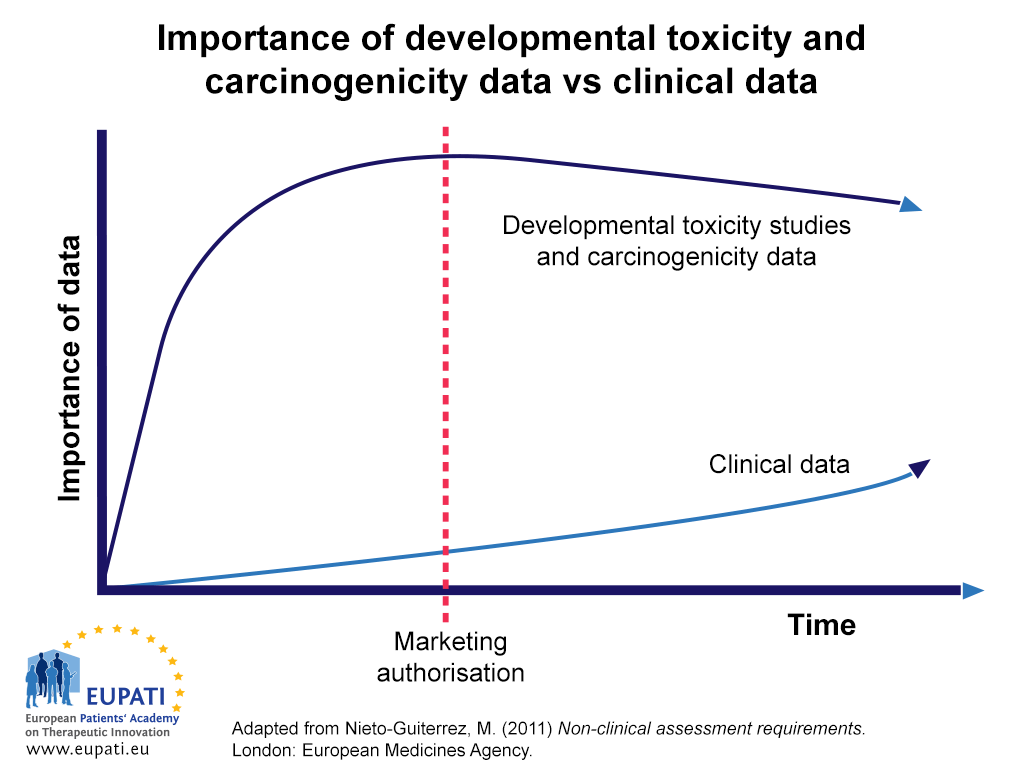
- Data from developmental toxicity studies and carcinogenicity studies continue to be relied upon more than clinical data throughout development and after marketing authorisation.
Types of Toxicology Studies
The following kinds of toxicology studies must be performed during non-clinical testing:
- Systemic toxicology studies
- Reproductive toxicology studies
- Male fertility studies
- Female reproduction and developmental toxicology studies
- Local toxicology studies
- Hypersensitivity studies
- Genotoxicity studies
- Carcinogenicity studies
These are explained in turn in the sections below.
Systemic toxicity studies
Systemic toxicology studies investigate the toxicity profile of the candidate compound in all of the animal’s tissues and organs. Systemic toxicology studies can be either single-dose or repeated-dose studies.
Reproductive toxicity studies
Reproduction toxicity studies investigate the effect of the candidate compound on the ability to reproduce and develop normally. These studies should be conducted as is appropriate for the population to be exposed to the candidate compound, and according to the following considerations:
- Men can be included in Phase I and II clinical trials before the conduct of the male fertility study, as an evaluation of the male reproductive organs is performed in the repeated-dose toxicity studies, although these studies should happen early in the process whenever possible. In any case, a male fertility study should be completed before the initiation of large scale or long duration clinical trials (for instance, Phase III trials).
- Women not of childbearing potential (for instance, permanently sterilised or postmenopausal women) can be included in clinical trials without reproduction toxicity studies, if the relevant repeated-dose toxicity studies (which include an evaluation of the female reproductive organs) have been conducted.
- If women of child-bearing potential are identified as a potential user population of the medicine, reproduction toxicity studies need to be done as early as possible.
Local tolerance studies
Local tolerance studies investigate the effect of the compound on the skin or eyes. These local toxicity studies are usually part of the general toxicity studies. To support limited human administration by non-therapeutic routes, e.g. a single intravenous dose for determination of absolute bioavailability, a single dose local tolerance study in a single species is usually sufficient.
Genotoxicity studies
Genotoxicity studies investigate the effect of the candidate compound on the chromosomes and genes, and are generally needed to support human safety. Assessment of gene mutation is considered sufficient to support all single-dose clinical trials. For multiple-dose clinical trials, an additional assessment of chromosomal damage in mammalian systems is needed, and a full battery of tests for genotoxicity should be completed before initiation of Phase II clinical trials. If positive findings are observed in genotoxicity tests, the need for additional testing must be considered.
Carcinogenicity studies
Carcinogenicity studies assess the effect that the candidate compound has on cancer generation. Carcinogenicity studies are generally conducted to support the marketing application of a new medicine. However, if there is a significant cause for concern, carcinogenicity studies should be conducted to bolster safety within clinical trials. In this case, a longer-term clinical trial duration with frequent monitoring, can be carried out. Generally, for medicines indicated for serious diseases in adults or paediatric patients, carcinogenicity testing may be concluded post-approval, based on the assumption that the early access to the medicines for patients outweighs the possible risk, although the earlier these tests can be completed, the better.
Attachments
- Presentation: Non-Clinical Development
Size: 478,517 bytes, Format: .pptx
Presentation on aspects of non-clinical development, including its aims, background activities, and the different types of non-clinical study.
A2-2.02.4-v1.3