Last update: 13 December 2016
Introduction
This EUPATI Mini-course starter kit is designed for patient involvement in product information, informed consent and patient information to trial participants.
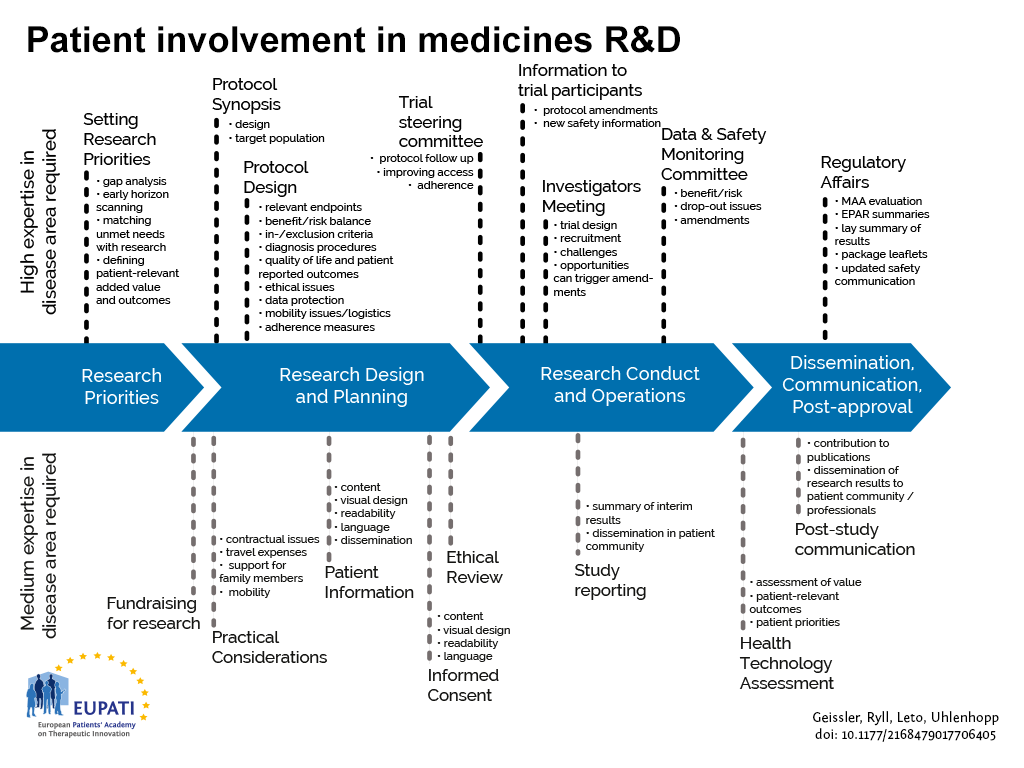
EUPATI Mini-course starter kits have been derived from content found in the EUPATI toolbox and EUPATI Patient Expert Training Course. The starter kits are thought to address roles that patients play in medicines development for example those shown in the figure below.
- Patients can be involved across the process of medicines R&D. This diagram created by Geissler, Ryll, Leto, and Uhlenhopp identifies some existing areas in which patients are involved in the process. It distinguishes between the level of expertise in a disease area that is required and the different areas where involvement can take place.
The starter kits provide you with links to relevant background reading in the toolbox and associated PowerPoint slide decks and media in order to prepare a single or multi-day training on the subject. Each of the starter kits contains a selection of PPT slides which you may use to educate patients/advocates about the “basics” in that area, e.g. in a two-hour to one-day seminar.
The starter kits are based on existing content from the EUPATI Toolbox, plus additional links to add-on Toolbox material. None of the “starter kits” are “ready-made course” modules – they are a ready-to-reuse resource for an experienced trainer to prepare and execute a course. You will need to edit them and put them into context.
Before you begin please download and review the ‘Manual for Trainers’.
- Presentation: Manual for Trainers
Size: 722,143 bytes, Format: .pptx
A manual for trainers describing how to use the EUPATI mini-course starter kits to create trainings on patient involvement.
Product information, informed consent and patient information to trial participants
This starter kit provides background reading, slides, a video, and quizzes to create training for patients who intend to become involved in product information, informed consent and patient information to trial participants.
Core reading
Risk communication in medicines
Information on medicinal products
Participants rights responsibilities organisations
Recording and reporting of clinical trial results
Clinical study results publication and application
Presentations
- Risk-Communication-in-Medicines
Size: 495,341 bytes, Format: .pptx
A presentation describing risk communication process in medicines, which can be adapted for own use.
- Presentation: Information on Medicinal Products
Size: 547,126 bytes, Format: .pptx
A presentation describing the information in medicinal products, which can be adapted for own use.
- Presentation: Participants’ Rights, Responsibilities and Role of Patient Organisations
Size: 387,072 bytes, Format: .pptx
A presentation describing the clinical trial participants’ rights, responsibilities and role of patient organisations, which can be adapted for own use.
- Presentation: Recording and Reporting Clinical Trial Results
Size: 372,913 bytes, Format: .pptx
A presentation describing the recording and reporting of clinical trial results, which can be adapted for own use.
- Presentation: Clinical Study Results Publication
Size: 449,072 bytes, Format: .pptx
A presentation describing the clinical study results publication, which can be adapted for own use.
Additional Learning Resources
Are you ready to elevate your expertise with certifications in areas like Clinical Development, Medicine Discovery, and HTA Evaluation?
Explore the links below to access the EUPATI Open Classroom, choose the courses that match your interests, and embark on your learning adventure today!
- Explore Clinical Trials and delve in the Rights & Obligations of trial participants!
- Discover the Basics of Interpreting and Disseminating Clinical Trial Results!
- Deepen Your Understanding of Medicinal Products!
- Grasp key concepts of Risk Communication used in Medicines Development!
- Navigate the Landscape of Regulatory Affairs!
- Curious about Marketing Authorisations? Find out more!
Videos
Informed consent for vulnerable populations (Webinar)
Terms of use - Creative Commons
Remember that all educational content provided by EUPATI is released under a Creative Commons License, which also applies to all derivatives of it! You can read more about the use of EUPATI content on the Creative Commons page.
Use of the EUPATI logo
The EUPATI logo is protected by trademark and owned by the European Patients Forum.
Except for the limited purpose of indicating that work is created or licensed by EUPATI (European Patients Academy for Therapeutic Innovation), or collaboration with EUPATI, the European Patients Forum (EPF) does not authorise the use, by any party, of the trademark "EUPATI" or any related trademark or logo of EUPATI without the prior written consent of EPF. Any permitted use will be in compliance with EUPATI's then-current trademark usage guidelines, as may be published on its website or otherwise made available upon request from time to time.
A2-SK-product-information-informed-consent-patient-information-to-trial-participants-V1.0