The EUPATI Patient Involvement in Medicines R&D Roadmap Video
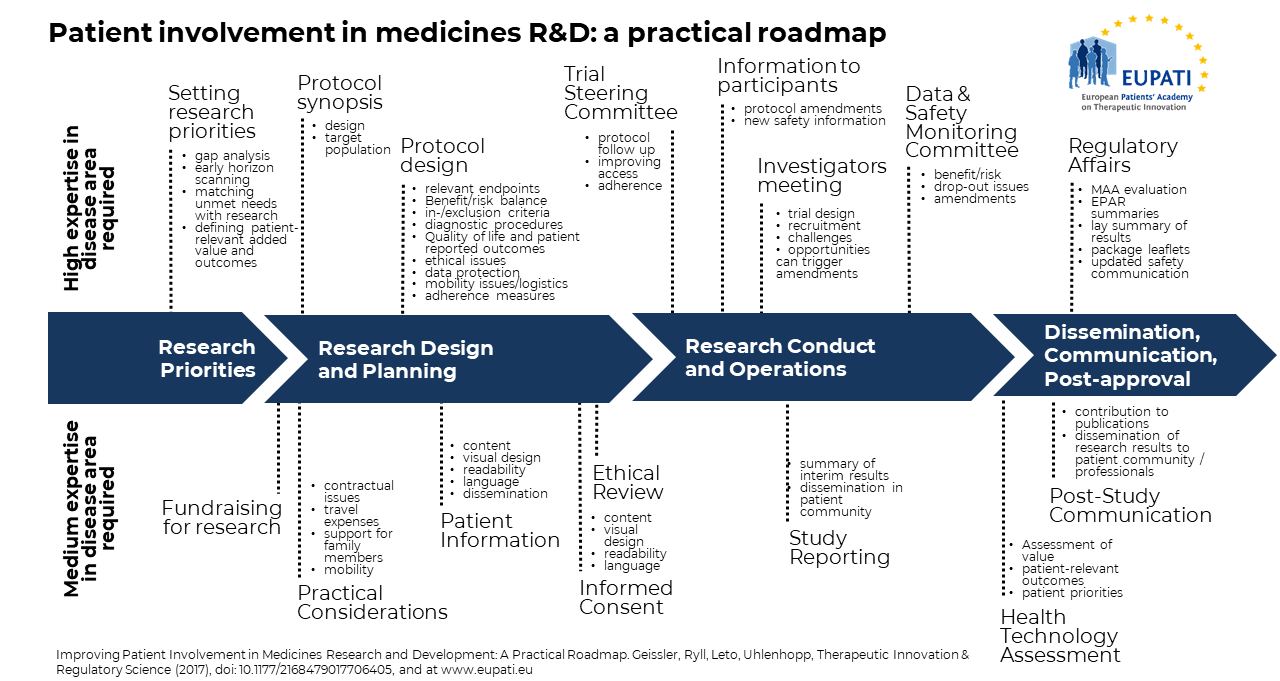
What is the EUPATI Patient Engagement Roadmap?
The value of patient involvement in medicines research and development is increasingly recognised by all stakeholders. Unfortunately, limited formal documentation of patient involvement activities hampers the sharing of experience and learnings, preventing timely and systematic implementation. Patient involvement often lacks structure and consistency in approach and happens too late. An end to end, practical guideline is required and the EUPATI Patient Engagement Roadmap, a process model for patient involvement in medicines Research and Development, provides this. Please see the image above (Source: Geissler, J., Ryll, B., Leto di Priolo, S., Uhlenhopp, M.: Improving Patient Involvement in Medicines Research and Development: A Practical Roadmap. Therapeutic Innovation & Regulatory Science 2017.)Using the EUPATI Patient Engagement Roadmap
The roadmap highlights specific opportunities for patient involvement along the four key stages of the medicines Research and Development lifecycle and is illustrated with concrete examples. This roadmap’s aim is to provide a tool to facilitate patient involvement during this lifecycle and is being shared to encourage implementation and further refinement. The roadmap intends to stimulate further discussion. All involved parties, academia and pharmaceutical industry, patient organisations and patients, clinicians and researchers, will need to be involved in the identification of strategic patient involvement points and their implementation to maximise the benefit for all stakeholders.EUPATI's commitment to the implementation of the Roadmap
The question of successful implementation is an important challenge, as the Roadmap and the Guidance Documents need to be put into practice by the different stakeholders. Involving patients in research can hugely benefit the medicines development process: by bringing in their priorities and perspectives, patients can contribute to developing better treatments for them and others. Greater patient involvement in R&D will boost the efficacy and safety of new treatments and increase public support for medical research. To enable this, it is essential that patients have thorough knowledge of the processes and methods by which medicines are developed and brought to the market to understand where and how they can make a meaningful impact. EUPATI’s long-term objectives remain focused on rigorous content development for patient education, the furthering of advocacy skills of patient experts, and the strengthening of a European patient movement. EUPATI is also widening its scope of activities to include training towards other stakeholders, e.g. patient engagement training for professionals working in industry and academia.In addition, the EUPATI Guidance Documents cover the interaction with regulatory agencies, HTA bodies, ethics committees and the pharmaceutical industry when aiming to interact involve patients in medicines research and development. There are four separate guidance documents covering patient involvement in Pharmaceutical industry-led medicines R&D, Ethics committees, Regulatory processes and Health technology assessments. Not only provide they practical guidance on how to interact effectively and transparently, but they also help to define more clearly what kind of input from patients is required – individual input with anecdotal patient experience, input from patient advocates who oversee the needs of a larger group of patients, formal positions of patient organisations, or input from patient experts who, in addition to aforementioned insights, also have the technical skills and training.
