Last update: 3 November 2020
Authors: Oyiza Momoh, Susan W. Burriss, Anya Harry, Kay Warner
The importance of inclusion and diversity in clinical trials
It is important that people participating in clinical trials truly represent the populations that scientific evidence shows are affected by the disease. This does not always happen. Patients from different backgrounds are not always brought into discussions about the plans and design of clinical trials (e.g. what information should be collected about the disease and how it affects them, or how the information is collected). This can mean the burden of participating in a clinical trial on patients is high and they may be put off from taking part. What’s more, if the patients reviewing the plans, designs and taking part in clinical trials are not from diverse backgrounds, the results of a trial may not be broadly applicable to all affected by the disease. This may lead to restrictions on the labelling of a medicine. Healthcare practitioners may feel that the information for use determined by the competent authorities does not reflect the population they want to treat, and they are therefore uncertain about how to use the medication.
For example, imagine four people:
- A 25-year-old, Indian, man, who likes to run regularly and lives in Zermatt, Switzerland
- A 50-year-old, Black, woman, who has a family history of high blood pressure and lives in Paris, France
- An 80-year-old, White, man, who smokes, has lung cancer and lives in Warsaw, Poland
- A 40-year-old, Asian, woman, who is pregnant with her first baby and lives in London, UK
These people may respond to medicines very differently because of personal risk factors; generally categorised into the following groups: behavioural; physiological; demographic; environmental and genetic.
Some factors affecting an individual’s health can change (e.g. lifestyle factors like running and smoking or environmental factors like the level of pollution we are exposed to). Other factors are those we cannot change (e.g. biological sex, genetics and ethnicity). These factors make us diverse and are further described in Figure 1. More information on risk factors in health and disease can be accessed via the EUPATI article here [1].
Classification of intrinsic and extrinsic factors
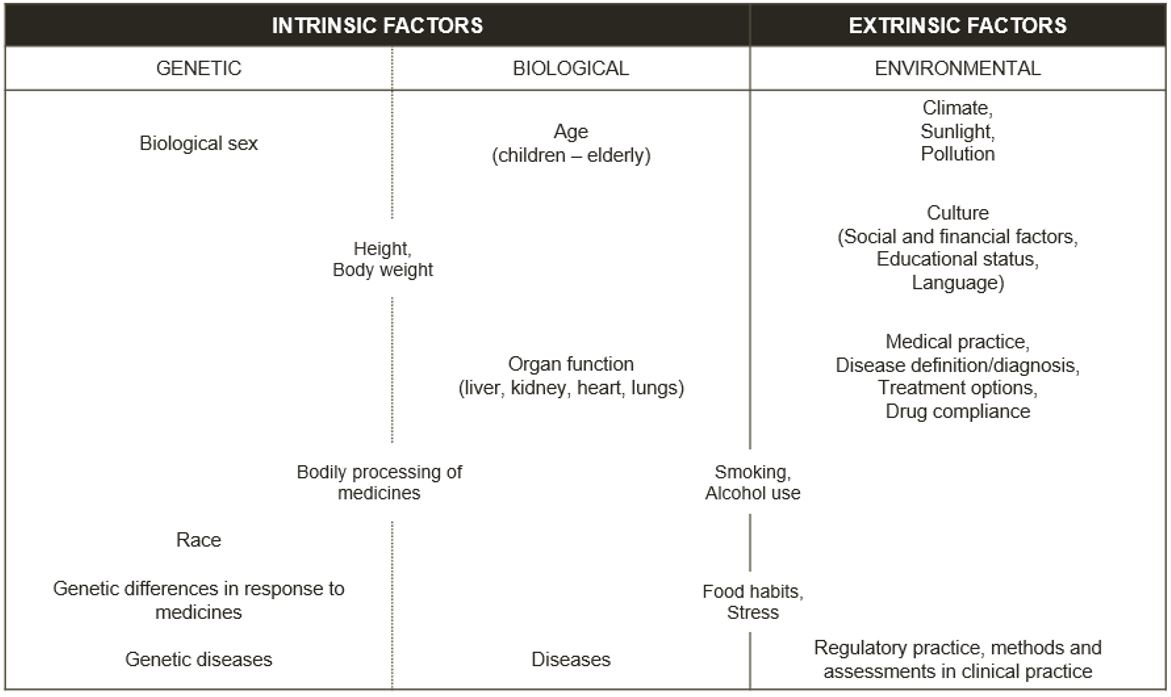
Figure 1 – Current standard for classification of intrinsic and extrinsic factors [2]
Genetic studies guide researchers on the biological basis of potential medicines. The scientific journal, Nature, reported that 96% of patients included in genetic studies for Alzheimer’s Disease and Type 2 diabetes between 2000 and 2009 were of European ancestry. By 2016, 81% were of European descent but only 0.08% were of Arab or Middle-Eastern descent [3]. By not involving more patients from other ethnic groups, we may miss out on the science that can give us crucial information about these diseases and how best to develop medicines for them. Instead, we may think that the results from a largely European group apply to all other ethnicities, which is not always true.
In the UK, the 2011 Census found that 14% of people were from Black, Asian and minority ethnic (BAME) backgrounds. However, UK data shows that 34% of critically-ill coronavirus patients are from BAME backgrounds [4]. This suggests that these people are more affected by the virus and/or have greater exposure than expected and clinical trials should include them to understand why. Unfortunately, clinical trials have not always included people from different groups, such as women, people over 65 or people from a range of ethnic backgrounds.
Barriers to participation in clinical trials by diverse participants
Current research shows there are common barriers to conducting clinical trials in different groups of people. They have been described as:
- Lack of trust in the pharmaceutical industry
-
- There may be cultural or age-related differences in how patients interact with healthcare systems because of past cases of unethical treatment of patients (e.g. the Tuskegee Syphilis Study, conducted between 1932 and 1972 by the U.S. Public Health Service). [5]
- Patient awareness of clinical trials and access to them
- Researchers may not be putting out the right communication to reach the patients they want to enrol in the clinical trials.
- Sites may not be local to patients or patients may not be able to travel to the places where clinical trials are being conducted.
- The trial documents (e.g. informed consent forms) may not be available in the necessary languages or might be written using complex terms.
- Site selection and engagement
- Patients from diverse populations may not be willing to participate, if the individuals who are managing the consenting process do not represent similar groups [6].
- There may also be investigator bias when asking participants to take part in clinical trials [7]. On one hand, sites may be more likely to contact their existing patient pool, which may not represent a diverse population. On the other hand, they may not adapt their recruitment process to suit diverse populations (e.g. involving families or community groups in the recruitment process) [8].
Overcoming the barriers to participation in clinical trials by diverse participants
Patient communities and organisations are vital to finding solutions to these barriers. This can be achieved by working with researchers to ensure the most suitable patients are being engaged to build trust, review materials and represent the voice of patients. Examples include:
- Building trust in the pharmaceutical industry
- More open communication and engagement between all stakeholders should be encouraged to build trust over time.
- Improving patient awareness of clinical trials and access to them
- Patient organisations that represent broad groups should be involved in the planning of clinical trials.
- Researchers should engage patients from different backgrounds in the review of clinical trial design and associated documents. This would ensure researchers obtain input from the people representing the appropriate target/real-world population for the clinical trial to help answer important questions (e.g. is there a financial burden of taking part in clinical trials? Are there study sites near the patients who represent the appropriate demographic population for the clinical trial? Are additional translations of documents needed to make them understandable for patients and facilitate participation?).
- Site selection and engagement
- Researchers could be advised on the barriers from a patient perspective (e.g. are the needs of people over 65 in Europe or in minority communities different to the US? How can researchers build community connections with diverse groups of people?).
Conclusion
Science should drive who takes part in clinical trials to ensure the appropriate patients are studied. Despite the barriers, regulatory agencies, researchers and patient organisations are beginning to focus on including diverse groups of people in clinical trials.
- In 2016, the US Food and Drugs Administration (FDA) described its recommendations and expectations of organisations that run clinical trials to collect and report ethnicity data for regulatory submissions [9].
- In 2019, a collection of pharmaceutical companies from around the world (TransCelerate Biopharma) introduced the Patient Experience Initiative, which aims to improve engagement and partnership between patients and the industry.
- In 2020, EUPATI Belgium published an in-depth review of potential solutions to these barriers [10].
Simply put, improving the diversity of participants in clinical trials will benefit everyone. However, this is not a simple ask, as demonstrated by the latest FDA Drugs Trials Snapshots [11]. When there is a high disease burden in a given demographic that remains underrepresented, Sponsors are often asked to do a post-marketing commitment study that increases the cost and timeline of the program. A clear example of this is the inclusion of women in clinical trials. HIV and cardiovascular clinical trials still do not match the proportion of female participants with the proportion of females with these conditions [12]. Treatment of female patients based on results from males may lead to unexpected side effects because of the differences in the way people process medicine and the way diseases affect them [13]. The risk of these side effects appearing may be reduced if the appropriate patients are involved in clinical trials. Therefore, data on the safety and effectiveness of medicines in different groups of people (e.g. by age, sex and ethnicity) can benefit broad populations. The data can provide information to assist treatment decisions, showing if the medicine acts differently in various groups of people. Many barriers do need to be overcome to achieve this. For example, sites may need Principal Investigators and other staff that represent diverse genders, racial and ethnic backgrounds. They may also need improved recruitment materials and an ability to translate documents to the languages of the patient population [14]. With increased input from a diverse group of patients, researchers will improve their clinical trial designs and recruitment strategies to lessen the burden on patients and ensure trials are available to those that science tells us are most affected by the disease. Discussions between patient groups, researchers and regulators are crucial to keep sharing learnings and improve the landscape for all patients.
The patient community continues to be an important partner in addressing the barriers to clinical trial diversity. Patient organisations working together with researchers will ultimately benefit society in the mission to get safe, effective and high-quality medicines to all the patients who need them.
- EUPATI, 2015. Risk factors in health and disease. https://toolbox.eupati.eu/resources/risk-factors-in-health-and-disease/ [Accessed 4 July 2021]
- Adapted from Guideline, ICH Harmonised Tripartite, 1998. Ethnic factors in the acceptability of foreign clinical data E5 (R1). In International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use.
- Popejoy, A.B. and Fullerton, S.M., 2016. Genomics is failing on diversity. Nature News, 538(7624), p.161.
- National Institute for Health Research, 2020. NIHR and UKRI launch call for research on COVID-19 and ethnicity [online]. Available at: https://www.nihr.ac.uk/news/nihr-and-ukri-launch-call-for-research-on-covid-19-and-ethnicity/24658 [Accessed 23 April 2020].
- EUPATI, 2015. https://toolbox.eupati.eu/resources/ethics-in-human-medical-research/
- Society for Clinical Research, 2018, Recruiting Diverse Patient Populations in Clinical Studies: Factors That Drive Site Success.
- Clark, L.T., Watkins, L., Piña, I.L., Elmer, M., Akinboboye, O., Gorham, M., Jamerson, B., McCullough, C., Pierre, C., Polis, A.B. and Puckrein, G., 2019. Increasing diversity in clinical trials: overcoming critical barriers. Current problems in cardiology, 44(5), pp.148-172.
- George, S., Duran, N. and Norris, K., 2014. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American journal of public health, 104(2), pp.e16-e31.
- Food and Drug Administration, 2016. Collection of race and ethnicity data in clinical trials. Guidance for Industry and Food and Drug Administration Staff https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials [Accessed 15 May 2020].
- Grine, L., Janssens, R., van Overbeeke, E., Derijcke, D., Silva, M., Delys, B., Dusart, I., Aertsen, V., Mertens de Wilmars, M., Robaczewska, J. and Stevens, H., 2020. Improving Patient Involvement in the Lifecycle of Medicines: Insights From the EUPATI BE Survey. Frontiers in Medicine
- US Food and Drug Administration, 2019. Drug Trials Snapshots Summary Report. https://www.fda.gov/media/135337/download [Accessed 12 October 2020].
- Feldman, S., Ammar, W., Lo, K., Trepman, E., van Zuylen, M. and Etzioni, O., 2019. Quantifying sex bias in clinical studies at scale with automated data extraction. JAMA network open, 2(7), pp.e196700-e196700.
- Wallach, J.D., Sullivan, P.G., Trepanowski, J.F., Steyerberg, E.W. and Ioannidis, J.P., 2016. Sex based subgroup differences in randomized controlled trials: empirical evidence from Cochrane meta-analyses. BMJ, 355.
- Oh, S.S., Galanter, J., Thakur, N., Pino-Yanes, M., Barcelo, N.E., White, M.J., de Bruin, D.M., Greenblatt, R.M., Bibbins-Domingo, K., Wu, A.H. and Borrell, L.N., 2015. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS medicine, 12(12), p.e1001918.


