Last update: 11 July 2023
Introduction
An R&D collaboration between the European AIDS Treatment Group, European Liver Patients Association, Treatment Action Group, and Janssen R&D.
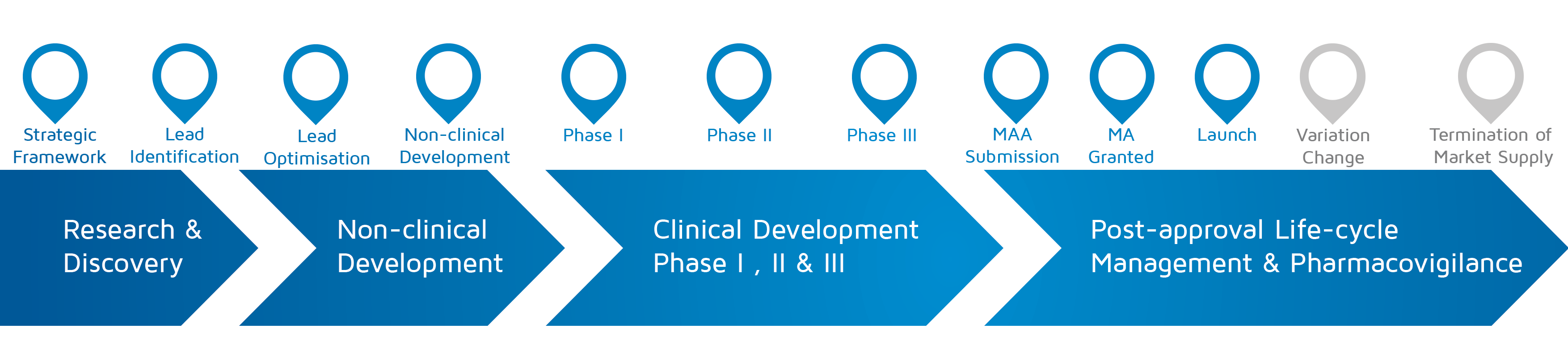
- When does it happen? – Strategic framework to Launch
Description of the case
Patients were involved as follows:
- Protocol design and review.
- Informed consent form (ICF) review.
- Participation in Drug Safety Monitoring Board (DSMB).
- Participation in Investigator Meeting.
- Building capacity in the area of Health Economics.
Janssen initiated also a collaboration with the London School of Economics and a tailor-made educational program was constructed for the EATG members. Afterwards, the course was handed over for further capacity building within their organisation without further company involvement.
Type(s) of patient (advocates) involved
- Patients / parents with personal disease experience.
- Expert patient / patient advocate with good expertise on disease, but little R&D experience.
- Expert patient / patient advocate with good expertise on disease and good R&D experience.
Benefits of patient involvement
Thanks to this collaboration we obtained:
- More targeted development.
- Better understanding of real needs for research and development.
- Faster study enrolment.
- Closer contacts between R&D experts and beneficiaries (motivational benefit).
- Better outcomes for patients.
Challenges and barriers
In disease areas outside HIV, professors and experts not always in favour of having patients on board.
We have discussed with them and showed EATG success example as model.
Learnings
Need to structure the process to ensure continuing process beyond individuals.
Patient literacy needs to be ensured to optimise feedback.
Ability to replicate this collaboration across more patient groups and advocates to increase knowledge on different topics, amongst other, Health Economics.
A3-HIV-V1.0
Attachments
- Case-Report-HIV_v1_EN
Size: 507,880 bytes, Format: .pdf
An infographic describing a patients involved case report on an R&D collaboration between the European AIDS Treatment Group, European Liver Patients Association, Treatment Action Group, and Janssen.