Last update: 15 July 2015
A biologic medicine is defined by the European legislation as ‘a medicine that contains one or more active substances made by or derived from a biological source.’1 In the broadest sense, biological medicines include any substance made in the laboratory from a living organism. This broad definition includes vaccines, immunotherapies, biosimilars, gene therapy, and stem cell or tissue therapy. In this article, we will use the term ‘biologic medicines’ to refer to protein-based medicines, such as insulin.
Biological or natural sources include micro-organisms, animal cells or human cells. Some biologic medicines mimic proteins made naturally in the human body. Examples include insulin, growth hormone, and growth factors that control blood cell production.
Other biologic medicines are not copies of proteins occurring naturally in the human body, but are enhanced in the laboratory to improve bioavailability, specificity, and effectiveness. The best-known examples of these are antibodies, which bind to the surface of cells in the body and are used extensively in the treatment of cancer.
Biologic proteins are much larger and more complex molecules than traditional chemical medicines; this means they cannot be manufactured as a pill, so they need to be administered via an injection.
Biologics are designed to have very specific effects and to interact with specific targets in the patient’s body, mainly on the outside of cells. A more targeted mechanism of action should lead to a greater chance of the medicine having the desired effect against the disease and should result in fewer side effects than traditional medicines. One common side effect of biologics, however, is the risk of immune reactions (immunogenicity), whereby the patient’s immune system recognises the biologic as a ‘foreign’ protein and tries to destroy it. This type of immune reaction may stop the biologic from working entirely or may just cause an irritation at the injection site.
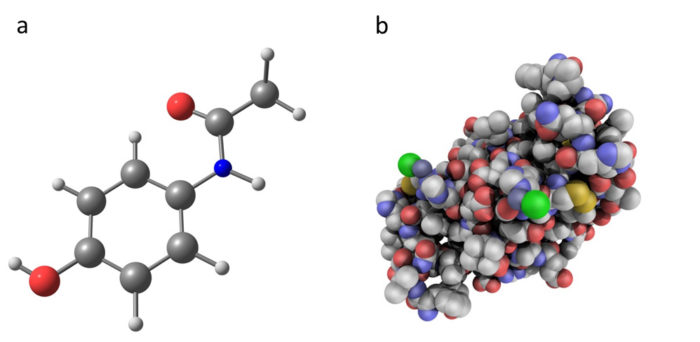
- Comparing paracetamol (a chemical molecule) with insulin (a biologic medicine). Paracetamol, as an artificially developed chemical molecule, has a much simpler structure than insulin, which is a protein-based biologic medicine. Biologic medicines contain larger and more complex molecules than traditional chemical medicines.
Further Resources
- U.S. Food and Drug Administration (2014, April 10). What is a biological product? Retrieved 5 July, 2021, from
https://web.archive.org/web/20171207134552/https://www.fda.gov/AboutFDA/Transparency/Basics/ucm194516.htm
References
- European Medicines Agency (25 Ocotober, 2019 last updated). Biosimilar medicines: Overview. Retrieved 17 July, 2021, from https://web.archive.org/web/20160315183115/http:/www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/document_listing/document_listing_000318.jsp
A2-1.06.4V1.2