Clinical phase
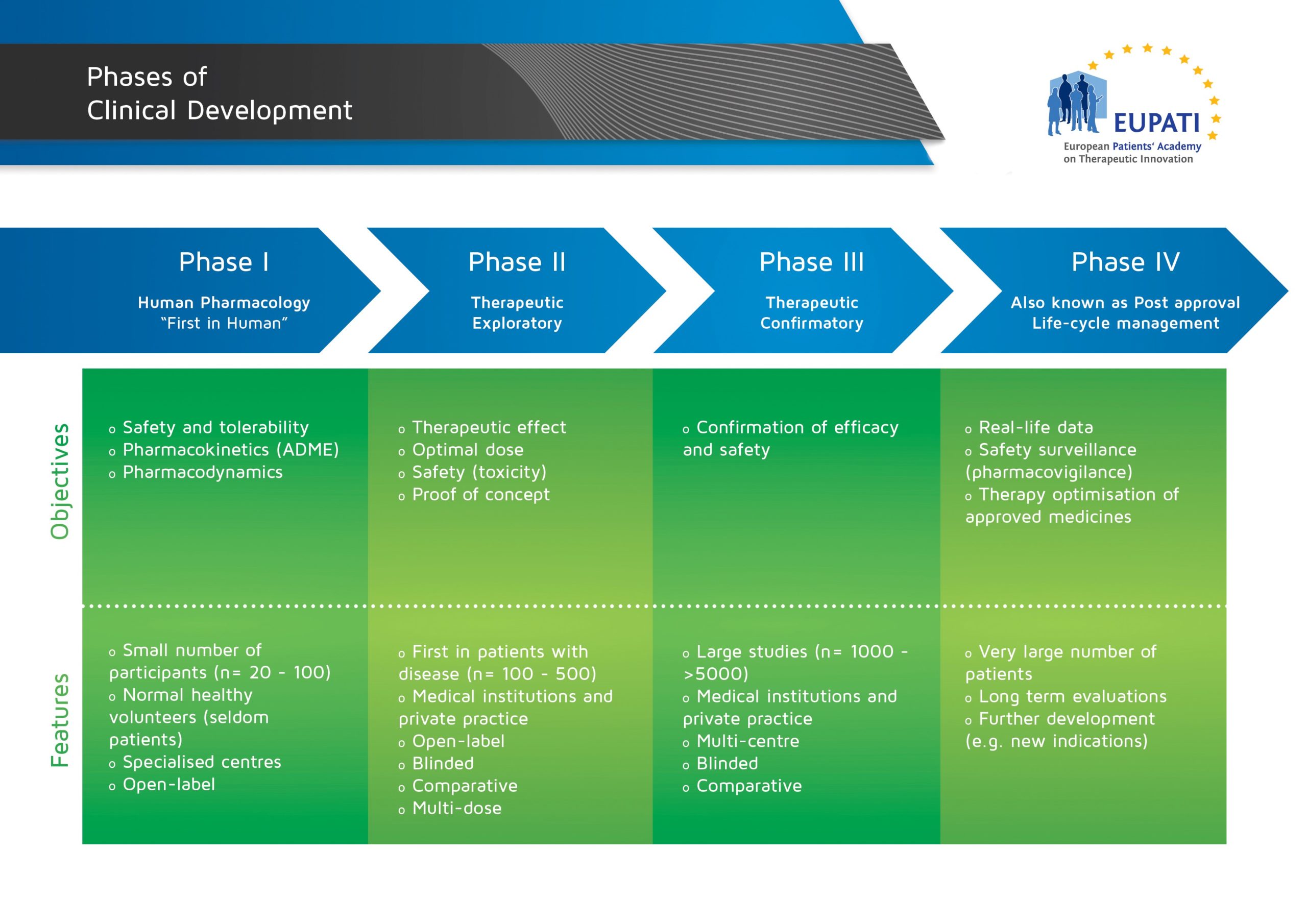
« Back to Glossary IndexThe clinical phase of medicines development is the one involving humans, and is different from the ‘non-clinical’ or ‘pre-clinical phase’ in which studies are performed in labs or in animals (such as for pharmacology/toxicology analysis). Clinical studies are conducted in four steps, called ‘phases’ – each designed to answer separate research questions:
- The four phases of clinical development differ in terms of their objectives and features.