Last update: 18 July 2023
Introduction
Involvement of European Tuberous Sclerosis Complex Patient Organisations (E-TSC) establishing an European registry of patients living with Tuberous sclerosis complex (TSC) to address some gaps in understanding the clinical course of TSC and the therapeutic outcomes.
Description of the case
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder with a birth incidence of 1 in 6000.
- It is a multisystem disorder characterised by benign tumours (hamartomas) that arise in multiple organs, including the brain, kidneys, skin, eyes, lungs, heart, and liver.
- Although considerable information on TSC has been obtained through recent research, gaps still exist in our understanding of the course of TSC manifestations and their prognostic role, rare symptoms and co-morbidities, interventions, treatments and their outcomes, and quality of life.
- Large-scale data on TSC are not available and longitudinal data are very limited. Hence, very little is known about the natural history of TSC across the lifespan.
- TuberOus SClerosis Registry to IncreAse Disease Awareness (TOSCA) has been designed to address some of these gaps by collecting data from patients across many countries worldwide.
Representatives of E-TSC were involved since the first meeting of investigators to design the registry.
Type(s) of patient (advocates) involved
- Expert patient / patient advocate with good expertise on disease but little R&D experience.
Benefits of patient involvement
There was a clear consensus to establish an European registry to address some of the gaps in understanding TSC by collecting data from patients across Europe.
Collaborative working with an academic steering committee, 3 representatives of E-TSC (UK, Italy, France) and the pharmaceutical company was a key component of the registry. TOSCA is:
- A multicentre, international disease registry.
- Designed to collect data from patients with TSC across many countries worldwide.
- Aims to address the gaps in understanding the clinical course of TSC and the therapeutic outcomes.
Benefits for E-TSC were:
- E-TSC has always felt that data collection is fundamental and should come before anything else.
- To date, quality information collected across the nation is severely lacking and problems encountered and similarities in cases may not be accessed and compared.
- By incorporating patients with TSC across the EU, knowledge and surveillance on a rare disease and orphan medicines will be increased, outcomes will be registered, and the effectiveness of treatment will be assessed.
- This registry could provide the healthcare professionals with an excellent instrument for research.
Challenges and barriers
The only challenge was identify inside the E-TSC the right representatives able to explain the point of view of patients. In this particular disease the representatives are all parents/caregivers.
The dynamic and communication style of the different board members who met to discuss the registry had to be adapted to the different scientific knowledge and understanding of the three components (investigators, company, and patient organisation’s representatives).
Learnings
The early involvement was positive, one part of the registry is dedicated to patient needs in particular:
- To create a TSC socio-economics specific questionnaire for the patients/family.
- To evaluate the impact of the disease in the real life of the involved families.
- To measure the quality of life of TSC patients using validated questionnaires.
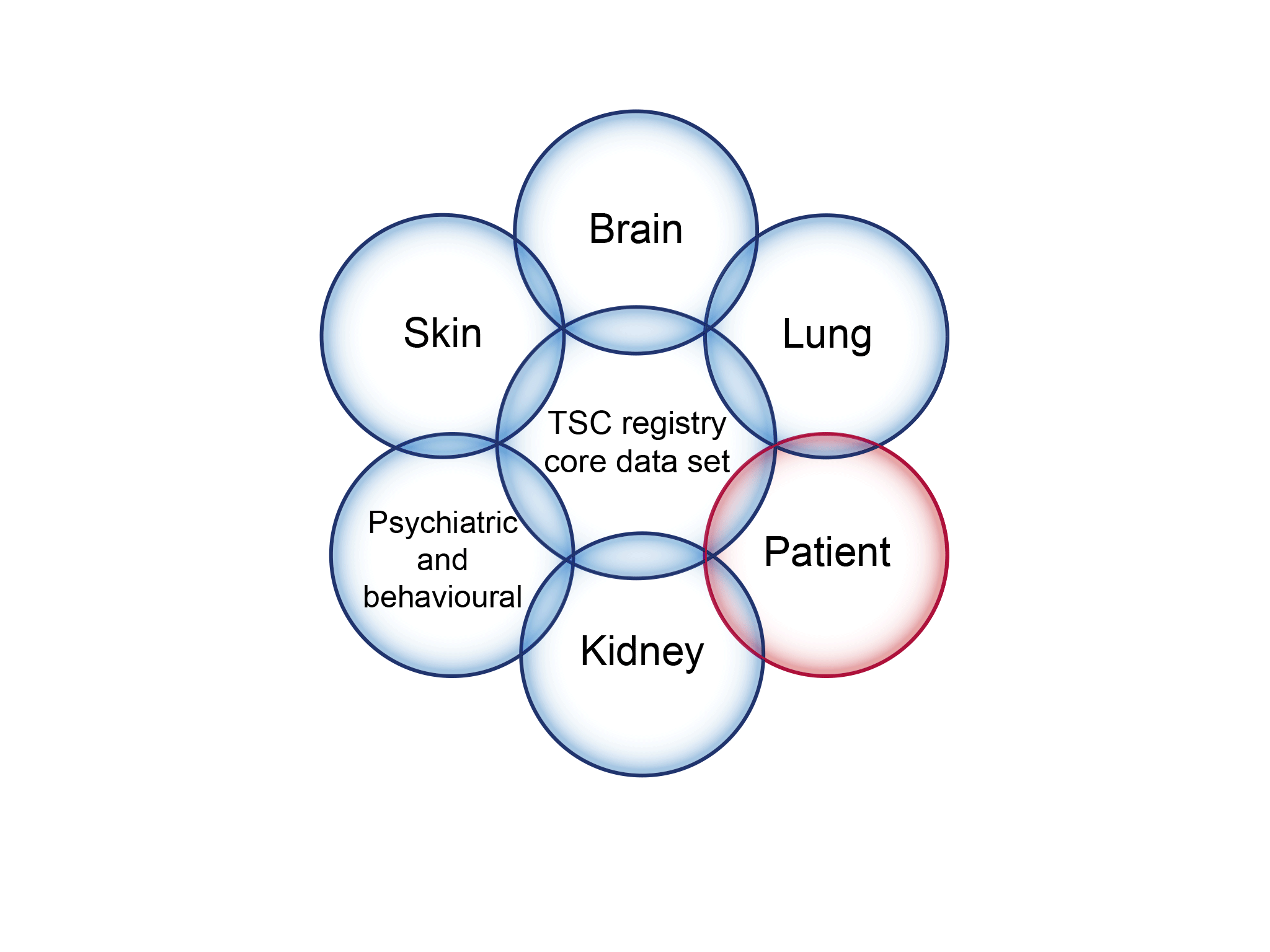
An illustration of the registry core data set to address knowledge gaps in the management of tuberous sclerosis complex (TSC).
It could be very useful in the future involve patient representatives already educated by the EUPATI Platform.
Attachments
- Patients Involved Case Report: Patient organisations’ input on a rare disease registry
Size: 1,148,541 bytes, Format: .pdf
An infographic describing a case of involvement of patient organisations establishing an European registry of patients living with Tuberous sclerosis complex (TSC).


