Last update: 11 July 2023
Introduction
Development of a cure/treatment for Duchenne, by the Duchenne Parent Project in collaboration with Leiden University and Prosensa (Biotech partner) – GSK.
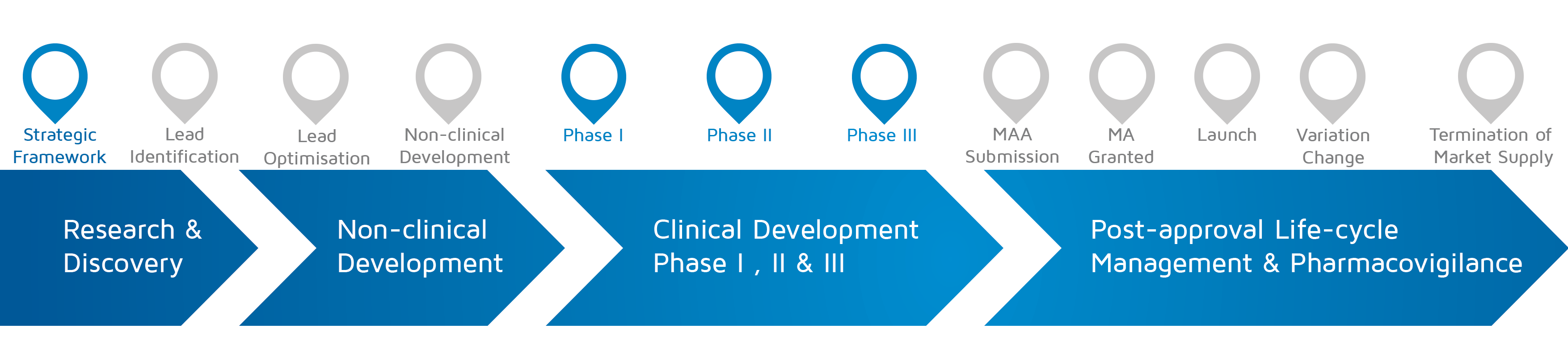
- When does it happen? – Strategic framework and Phase I-II-III
Description of the case
Development of a cure / treatment for Duchenne Muscular Dystrophy.
Duchenne Parent Project was involved in different aspects of the project:
- Funded research at the University (Leiden) and Biotech (Prosensa).
- Funded the Phase I b trial (local injection).
- Involved in recruitment and patient registries for the follow up trials.
- Involved in standards of care (needed for clinical trials).
- Involved in the development of outcome measures.
- Provided information to families and patients.
- Regulatory discussions.
Type(s) of patient (advocates) involved
- Patients / parents with personal disease experience.
- Expert patient / patient advocate with good expertise on disease and good R&D experience.
Benefits of patient involvement
Duchenne Parent Project (DPP) was part of the initiative from day one, without DPP the research project and follow-up probably had never started.
At the end medicines have to be proven ‘clinically meaningful’ to the patients, so starting from the patients is a very manner to develop medicines (bed to bench).
Patients are the driving force to speed up research and translation from the lab to the patient (bench to bed).
Challenges and barriers
Collaboration with the biotech company was ‘easy’. However, when the first product was taken over by ‘Big Pharma’ GSK, collaboration (such as discussions about trial design, outcome measures and recruitment and other policies) became very difficult as Big Pharma has strict rules not to interact with patients before a product is on the market. To have companies design trials for the full spectrum of patients and not only for a small label as at the end when the product comes on the market it is very likely only authorised for the same small label.
Regulators having very limited knowledge about the disease.
Learnings
In retrospective we should have started earlier with the collection of Natural History data. When you want a cure, collecting Natural History data doesn’t sound ‘sexy’, but it can really help speeding up the process of medicine development, cut down the size of the placebo group. When Natural History data are collected (and owned) by patient organisations they can be used by different companies.
Make sure you have outcome measures for all groups. We started and funding initiatives to develop these outcome measures.
Raise awareness among regulators about your disease and the preferences of the patients.
A3-DPP-V1.0
Attachments
- Case-Report-Duchenne-Parent-Project_v1_EN
Size: 493,493 bytes, Format: .pdf
An infographic describing a patients involved case report on the development of a cure/treatment for Duchenne Muscular Dystrophy.