Last update: 18 December 2023
The development of Medical Devices should involve patients and the public throughout each stage: innovation, assessment, implementation and evaluation.This is essential in order to ensure that Medical Devices address a need which patients and the public have identified & fit for purpose in terms of ease of use, acceptability, affordability and compatibility with other technologies. Currently, the development phase of Medical Devices does not promote active patient participation (in contrast to medicinal products), and thus the ability of patients to participate in the development of Medical Devices is quite limited.
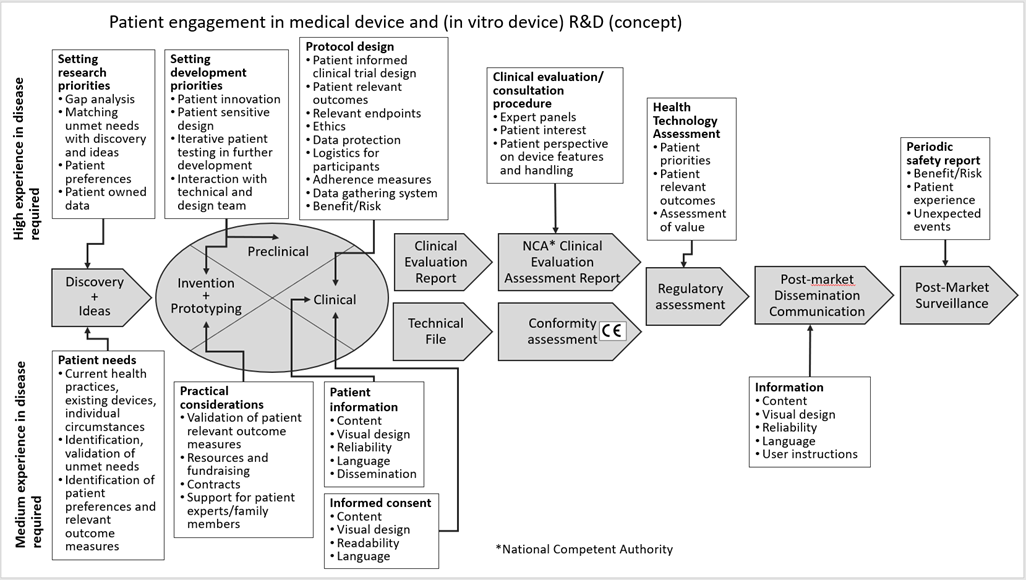 Figure 1: Concept roadmap of patient involvement in the different phases of the Medical Device R&D
Figure 1: Concept roadmap of patient involvement in the different phases of the Medical Device R&D
Patient Involvement Perspectives
Patients can provide highly valuable information that can contribute to the development of safe and efficacious medical devices, as well as educating and empowering other patients, thus enabling them to be more active in treating their disease and/or improving their quality of life.
Fostering Diversity and Inclusion in Decision-Making and R&D
Examining the steps in the development of Medical Devices and their lifecycle management from the manufacturer’s perspective, there are many instances where patient input can and should be sought.
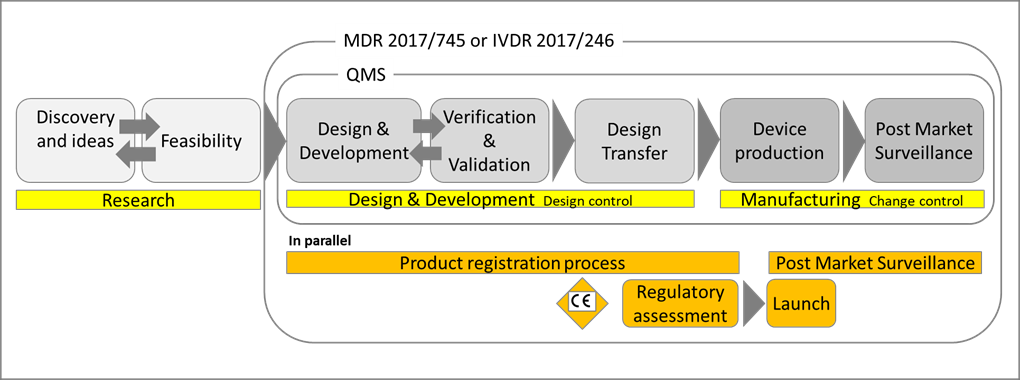
Figure 2: The phases of the Medical Device lifecycle [1].
It is also important that patient and public involvement is structured in a way which invites representation from as broad a range of the population as possible encompassing physical and cognitive ability or disability, gender, sex, age, race, ethnicity and socioeconomic background.
Surpassing Challenges in Market Access & Procurement
The CE marking process is designed to assess whether a device meets the safety, quality and performance standards laid down in the regulations before a device is made available to patients. Procurement is the act of obtaining goods or services, usually on a relatively large scale, i.e. by health care systems, to provide health services and products to patients. The involvement of patients throughout development, regulatory processes and final decisions does not have a prominent position in the process and thus whether or not a product becomes available tends to lean mostly on manufacturers’ and national decision makers’ individual policies.
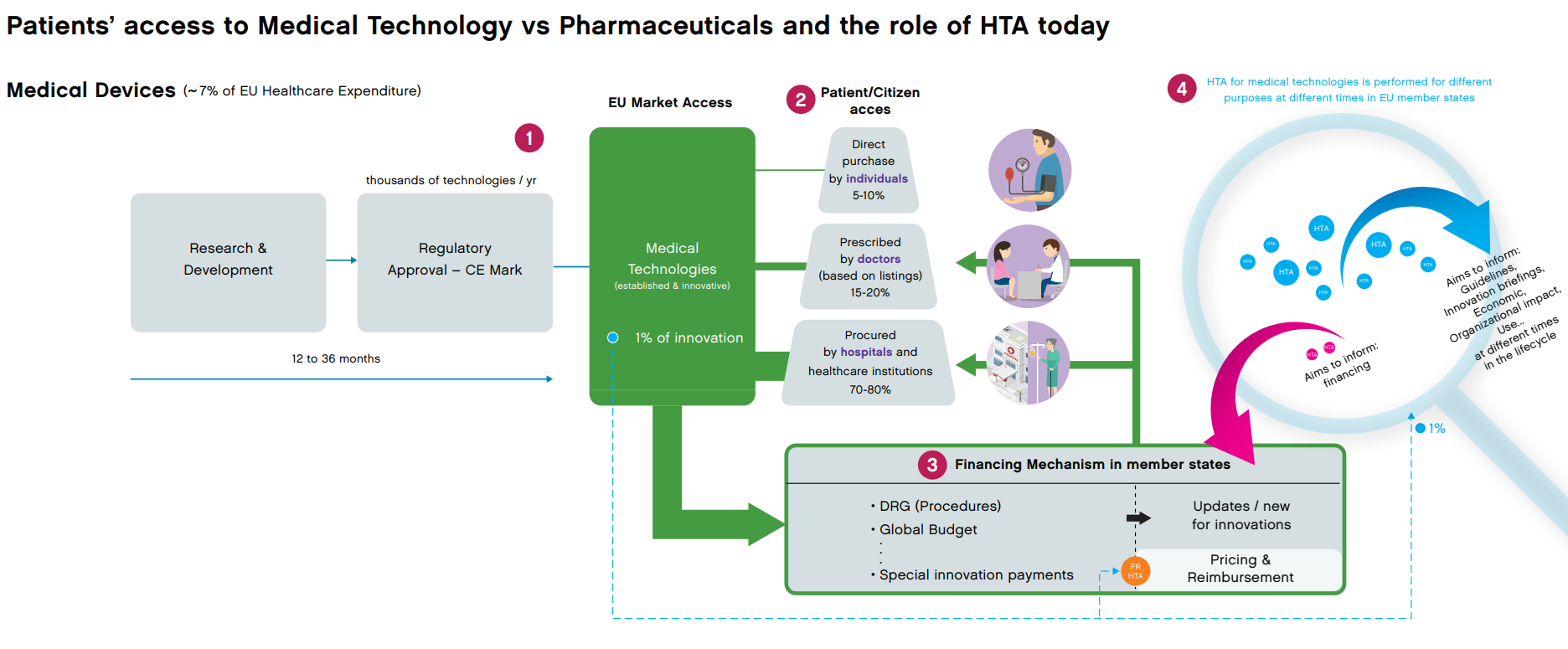
Figure 3: Medical and technology driven access model. MedTech Europe, Nov. 2020.
However, as these new models are being developed and implemented in value-based systems, there is an opportunity for patients to take an active role and make sure that the values reflect what is important for patients. This new approach to healthcare delivery is widely known as ‘Value-Based Healthcare’ (VBH), differs from a fee-for-service or tax-based system, and has been embraced as the best method for lowering healthcare costs (the total spending for the full cycle of care) while increasing health outcomes (functional recovery and quality of life), quality of services and helping people live healthier lives [2].
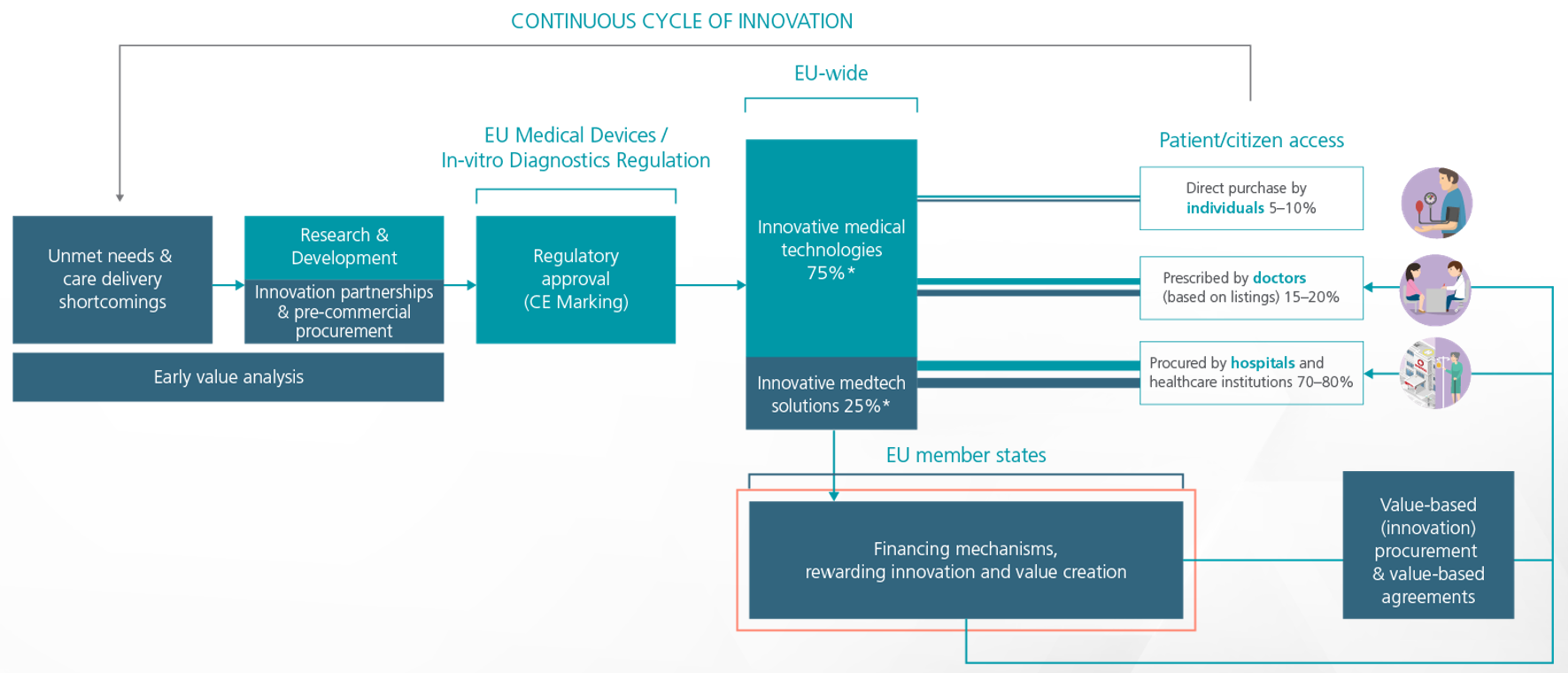
Figure 4: Value and innovation-driven access model. MedTech Europe, 2021.
Patient involvement in the context of Value-based healthcare should be understood at the system level, as patient involvement affects involvement in planning, design and development, as well as which projects the health system initiates or enters into, and which solutions are implemented either directly or via procurement or reimbursement schemes.
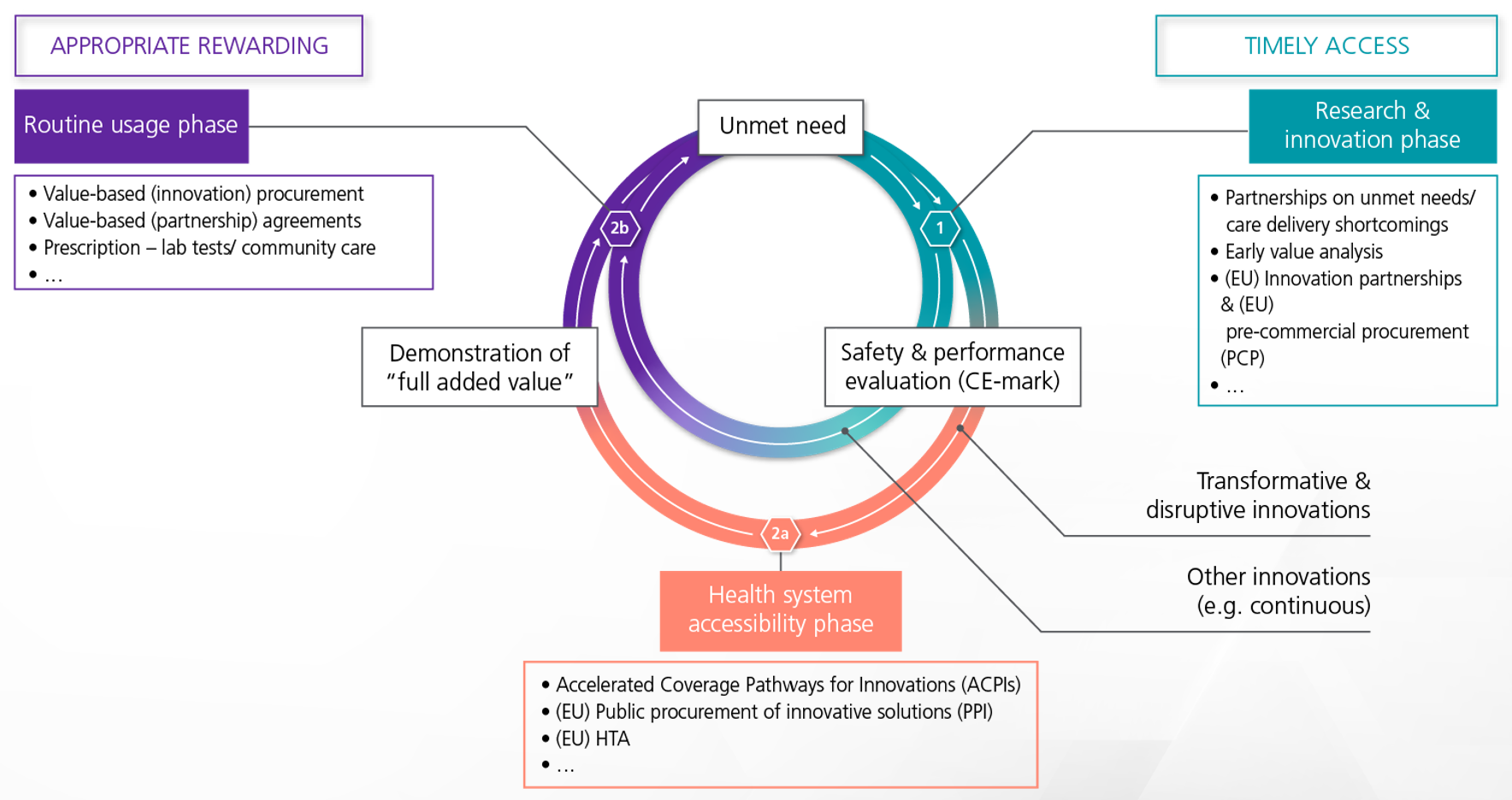
Figure 5: A patient-centered closed loop where patients play a key role in defining needs, value, safety criteria, HTA, and procurement processes. MedTech Europe, 2020.
Reshaping HTA Strategies
It is equally essential to involve patients where possible and appropriate in the HTA process. When planning the HTA questions and methodologies, patients should play an important role in introducing a continuum of operational aspects and pointing out challenges that should be assessed. Though not explicitly mentioned, patients can be also part of the expert panels, that are being voluntarily consulted by manufacturers, with the aim of reviewing the manufacturer’s intended clinical development strategy and proposals for clinical studies of Class III and some Class IIb medical devices during the clinical evaluation process. These expert panels shall also consider relevant information provided by patients’ organizations (MDR Article 106(4)).
Learning Resources
Take your skills to new heights by earning certification in emerging fields like Medical Devices! Explore the EUPATI Open Classroom and delve into the modules Medical Device development, Lifecycle management, New Technologies, Patient involvement, Introduction to market access, key elements and patient involvement & Introduction to Health Technology Assessment (HTA) of Medical Devices and IVDs to deepen your understanding of the Medical Devices’ landscape. Strengthen your expertise, empowering you to advocate for, engage in discussions, and play a pivotal role in implementing key elements that align with your vision for the healthcare system.
References
[1] From idea to post market surveillance: the phases of the medical device lifecycle. Wim Steenackers Business Development Manager at Quality by design; Medical Devices & IVD; April 26, 2021
[2] EIT. Implementing value-based health care in Europe – Handbook for pioneers. Source:
https://eithealth.eu/wp-content/uploads/2020/06/Implementing-Value-Based-Healthcare-In-Europe.pdf


