Last update: 30 June 2016
Introduction
The concept of ‘Quality of Life’ is crucial to patients and important for health technology assessment (HTA). It is also a difficult concept to measure. Although most people would say both length of life and quality of life are important, the meaning of ‘good quality of life’ may be different to different people.
What is quality of life?
There is no single definition of Quality of Life, though there have been many attempts to define it. Similar to their definition of health, the World Health Organisation (WHO) definition is among the more comprehensive definitions of quality of life. The WHO defines quality of life as:
‘…individuals’ perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns. It is a broad ranging concept affected in a complex way by the person’s physical health, psychological state, level of independence, social relationships, personal beliefs and their relationship to salient features of their environment’.1
The WHO suggests that quality of life encompasses several key areas, called ‘domains’.
These domains have items incorporated within them. See Table 1 below.
| Domain | Items incorporated within the domains |
|---|---|
| 1. Physical health (HRQoL) |
|
| 2. Psychological health (HRQoL) |
|
| 3. Level of independence (HRQoL) |
|
| 4. Social relationships (HRQoL) |
|
| 5. Environment |
|
| 6. Personal values and beliefs |
|
| Adapted from World Health Organisation WHOQOL-100.2 | |
Note that the first four domains of quality of life listed in the table above include aspects that may be directly affected by health and the use of medicines and healthcare technology, while the final two domains (environment and personal values and beliefs), although important, may not be as frequently affected by the use of health technologies (including medicines). This more narrow focus on the quality of life due to state of health is called ‘health-related quality of life (HRQoL)’.
Patients, payers or providers who want to understand the value of a treatment could include the last two domains, or assume they won’t change and then focus more specifically on aspects directly affected by health technologies.
As you can see from Table 1, HRQoL is multi-dimensional (contains multiple items and domains) including physical, psychological, functional, and social domains related to a person’s perception of quality of life affected by health status. It follows, then, that attempts to measure HRQoL will try to capture these domains.
The term HRQoL (also called HrQL, HRQOL, HRQL, QOL) has been widely adopted and promoted within the HTA community. The term HRQoL is used interchangeably with the generic term ‘quality of life’ as well as terms like:
- self-reported health
- patient-assessed outcomes
- patient-reported outcomes
- person-reported outcomes
- patient outcomes
- outcomes
HRQoL measures are types/subsets of patient-reported outcome (PRO) measures distinguished by incorporating different domains.
The terms ‘patient health status’ and ‘functional status’ have also been used to mean HRQoL, despite the fact that these measures do not necessarily require information from the patient’s perspective – that is, they are not necessarily PROs. Similarly, there also exist outcomes derived from information from parents, providers or caregivers about their perceptions of how a patient is feeling. These have been recently labelled observer-reported outcomes (ObservROs) and include clinician-reported outcomes (ClinROs).
Why measure Health-related Quality of Life?
There are many reasons why we might want to measure HRQoL:
- Patients and healthcare providers as well as payers are interested in the added value a technology (health intervention or use of health technology) has to offer. HRQoL can serve as a common measure of gains from any technology. Patient groups can use these measures to compare the values of new technologies. HRQoL measures are therefore often used in relation to their costs in an economic evaluation to support decision-making in HTA processes.
- HRQoL measures provide useful information to care providers as they can be used to screen and monitor patients for psychosocial problems or when auditing healthcare practice.
- HRQoL measures can be used in population surveys of perceived health problems or other aspects of health-services or evaluation research.
- Regulators can use HRQoL measures to help their assessments of new technologies.
For policy makers, who are supposed to decide how to allocate resources in healthcare, and HTA bodies, being able to appraise the value that a new technology may bring compared to other technologies across various types of patients is useful and may support their assessments or decisions. Payers are interested in science-based decisions and quantifying the gains that a treatment can provide for a patient. A generic instrument to measure HRQoL allows a numeric HRQoL score to be calculated. HRQoL measures allow HTA bodies to see quantifiable changes in patients’ well-being, but such instruments require qualitative research to design and develop.
Policy makers and HTA organisations may use such a numeric HRQoL score for instance in the calculation of a Quality-Adjusted Life Year (QALY) – although there are ongoing discussions about how to use QALYs in healthcare decision making or whether or not to use them at all.3
What is a Quality-Adjusted Life Year (QALY)?
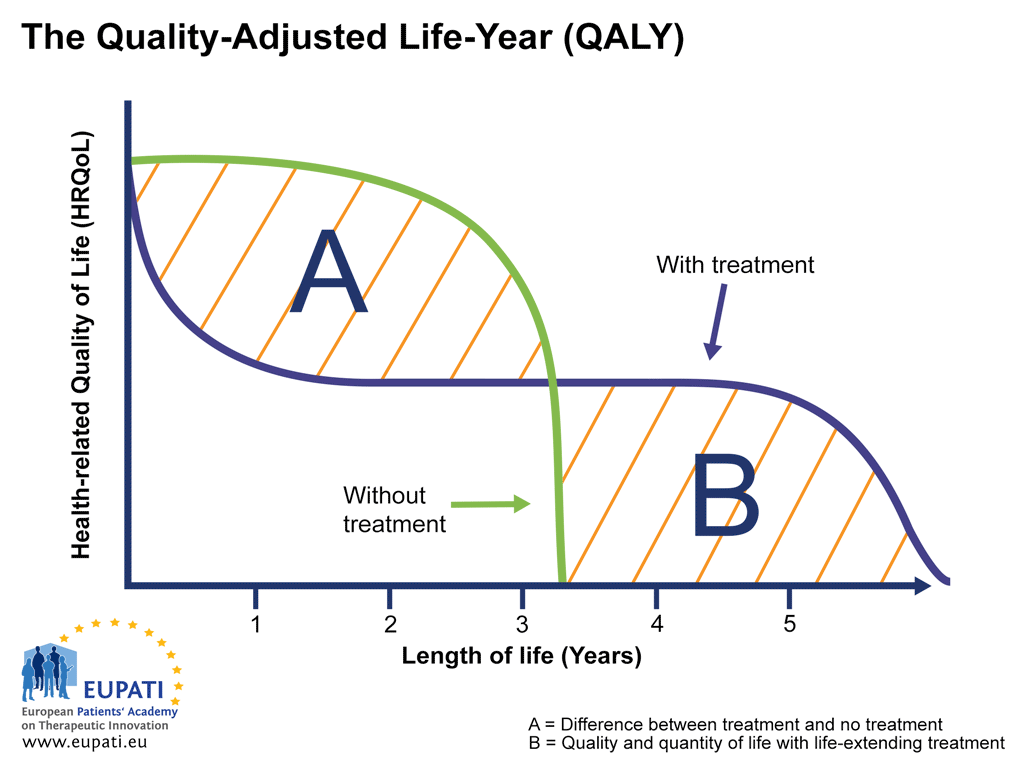
QALY attempts to represent the impact a therapy has on the length of life while also taking into account any changes in the health-related quality of life (HRQoL). HRQoL is calculated on a scale where 0 = ‘death’ and 1 = ‘perfect’ health (the scale also allows for negative scores).
Below is a sample calculation of QALY for a treatment that provides four years of perfect health:
Compare this with the calculation for a treatment that provides four extra years of life with an HRQoL score of 0.5:
QALY calculations can be used to visualise the relationship between the quality and quantity of life experienced with and without the therapy in question, as in the graph below.
- The Quality-Adjusted Life-Year (QALY) is a measure in health economics that expresses the additional number of years a person lives as a result of receiving treatment, taking into account the quality of life of those years.
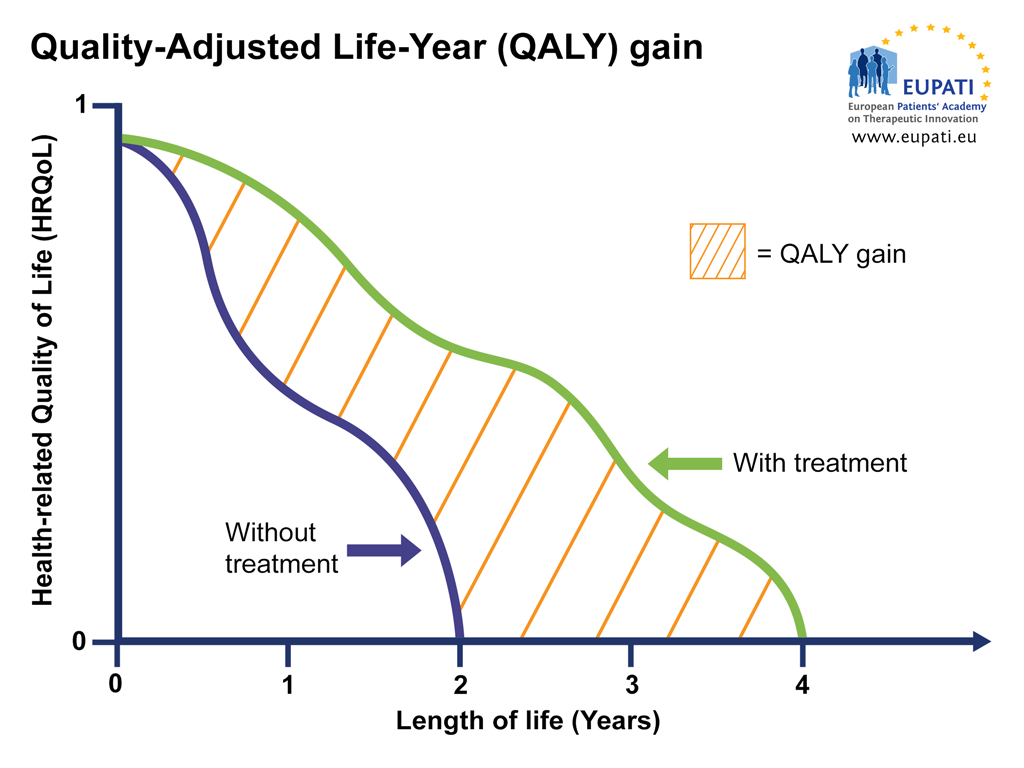
Similar graphs can be used to plot changes in HRQoL over time with and without treatment, providing a visualisation of the QALY gain or loss, respectively. In the graph below, for instance, the treatment provides an increase in HRQoL as well as an extension of life, resulting in a net QALY gain.
- The Quality-Adjusted Life-Year (QALY) gain of a patient receiving the treatment versus a patient who receives no treatment can be shown visually. The difference in area under the curve (AUC) represented by the orange area, shows the QALY gain between someone using the treatment versus someone who does not.
| Intervention | Life-years | HRQoL (score from 0-1) | QALY |
|---|---|---|---|
| No treatment | 2 | 1.0 | 2.0 |
| Treatment | 4 | 0.5 | 2.0 |
Reading 1: Reflection paper on the Regulatory Guidance For The Use Of Health-Related Quality Of Life (HRQL) Measures In The Evaluation Of Medicinal Products - https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-health-related-quality-life-hrql-measures-evaluation-medicinal-products_en.pdf
Note some concerns expressed by the regulator in assessing HRQoL. First, there is a concern about double-counting. We have seen in previous topics that the domains and items used to measure any PRO require validation and should not overlap. However, the regulator is more concerned here with scenarios where a new therapy both improves HRQoL and improves an outcome that is part of the HRQoL assessment (like reduction of pain).
They also suggest that companies who would like to claim improvements in HRQoL should show improvement across ‘all or most of’ several key domains’.
The last part of the paper devotes a considerable amount of attention to the proper use of these instruments in clinical trials. They advise those developing new medicines to have HRQoL instruments validated prior to conducting experiments. This avoids a situation of questionable data collection. We can imagine a situation where several different HRQoL instruments are developed and only one records an observable response to therapy. Is the response a property of the medicine or does it have something to do with the instrument? Evaluators are left with such questions if the validation of the instrument is not conducted prior to using it.
The paper also points out several other factors that may make interpretation of the findings from HRQoL measurement difficult. One is where patients know they are receiving therapy (an open label trial). Patients who know they are receiving therapy have been shown to be more positive when providing subjective information. It can be difficult for the evaluators to distinguish the effect of the medicine versus the satisfaction that the patient has from having access to something new (and believing it might be working).
A last point made is that although HRQoL is important, short-term measures do not really provide us with insights into a person’s overall well-being. Rather they tell us how patients are doing from day to day without providing information on how they might be over a longer and more meaningful period of time.
Current approaches to measuring HRQoL
There are many dimensions or domains related to HRQoL that need adequate methods of measurement. Simply asking a patient, ‘How is your health-related quality of life, on a scale of 1 to 10?’ provides limited information. Patients perceive and report the same conditions in different ways. Measuring HRQoL usually requires capturing various dimensions of what is important to patients.
For example, the overall answer to the simple question could be the same from day to day but does not consider that a person’s level of independence might be improving while psychologically they are deteriorating. That is, it may not distinguish between a severely depressed and very mobile patient and another patient who has very limited physical functionality but who is emotionally well. It should be considered that some domains (such as psychological vs physical functions) are valued more by patients than others, and this will be reflected in a patient’s reported HRQoL status.
As with any PRO, a tool used to capture HRQoL will ideally have the following properties:
| Properties required | Definition |
|---|---|
| Reliability | A reliable measure is consistent and reproducible |
| Test-Retest Reliability | Test-retest reliability examines the agreement among scores in stable persons at two points in time. |
| Internal Consistency | The extent to which items intended to assess health or functional status in a particular domain are correlated with each other and not correlated with items intended to measure other domains. |
| Intra- and Inter-Observer Reliability | The extent of agreement across assessments or among individuals. |
| Validity | The measure accurately reflects the concept it is intended to measure. |
| Content Validity | The extent to which the items are sensible and reflect the intended domain of interest. Does the content of the measure make sense? Are the items included relevant to the domain of interest? Do the items cover the full range relevant to that domain? Are the items comprehensible to respondents? |
| Criterion Validity | The extent of agreement between the measure and a gold standard measure of the same concept. |
| Construct Validity | A measure's ability to perform as expected. Evidence that the relationships among items and domains conform to a priori hypotheses and that logical relationships exist between the measure and characteristics of patients and patient groups. |
| Convergent Validity | Convergent validity refers to evidence of a moderate or strong relationship between measures of the same concept or construct. |
| Discriminant Validity | Discriminant validity refers to evidence of the lack of relationship between measures of a different concept or construct. |
| Cross-Sectional Construct Validity | Evidence of construct validity based on comparisons at a point in time. |
| Responsiveness (Longitudinal Construct Validity) | The ability of a measure to capture meaningful change when it occurs. |
| Interpretation | The ability to attach meaning to the scores provided by a measure. |
HRQoL is frequently measured with ‘tools’ in the form of questionnaires, for instance the 36 Item Short-Form (SF-36®) Survey5 or the EuroQoL 5 Domain (EQ-5D)6 tool. These tools are used extensively in the realm of economic evaluation and HTA, since their results can be converted to numerical values. This allows researchers to compare changes of HRQoL in one type of patient with those in other types of patients. More specific tools exist for certain disease areas such as HIV-QL31 for HIV or EORTC QLQ-C30 for cancer.
Like other PROs, the use of HRQoL measures in clinical studies must be done carefully. HRQoL instruments must be carefully planned and validated before the study begins, in order to avoid that instruments measure the wrong responses or misrepresent reality.
Alternative approaches to measuring HRQoL?
There are important dimensions of receiving care beyond simply duration and (health-related) quality of life. These include the quality of life of family and caregivers, and convenience for patients. Also, measurements may not consider unmet needs or distinguish between additional health gained by the very old or for the very sick (who may value small health gains to a greater extent).
The field of HRQoL measurement is rich with information and debate. While some propose new measures, others have suggested modifying existing ones. It is difficult to find consensus between various stakeholders about what should be measured as HRQoL, or how. Some systems face extra difficulties when discussing modifications if all their previous health system decisions were based on a single measure. The only consensus among experts is that it is preferable to invest in measurement only if a minimum level of quality of that measurement is guaranteed.
The dilemma is well expressed in the following quote by statistician John Tukey: ‘It is often much worse to have good measurement of the wrong thing especially when, as is so often the case, the wrong thing will in fact be used as an indicator of the right thing than to have poor measurement of the right thing.’
The role of patients
HRQoL measures are a type of patient reported outcome (PRO). Patients have opportunities to ensure that their perspective is considered in the development and design of these measures in the early stages of clinical development. They can also actively review and endorse HRQoL measures that meet quality standards and have included patients in their design and development.
Patients can also:
- scrutinise HRQoL measures and the magnitude of claimed changes in HRQoL used in HTA submissions and submissions for marketing authorisation to regulatory authorities,
- endorse certain HRQoL measures, and
- relate patient experiences in a way that reflects the key conceptual domains of HRQoL.
References
- World Health Organisation. ‘Introducing the WHOQOL instruments’. WHOQOL: Measuring Quality of Life. Retrieved 11 February, 2016, from http://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/
- World Health Organisation. ‘The Structure of the WHOQOL-100’. WHOQOL: Measuring Quality of Life. Retrieved 4 July, 2021, from https://web.archive.org/web/20200814200819/https://www.who.int/healthinfo/survey/whoqol-qualityoflife/en/index4.html
- ECHOUTCOME (2013). ‘EUCHOUTCOME Report Summary.’ European Commission: Community Research and Development Information Service (CORDIS). Retrieved 4 July, 2021, from https://cordis.europa.eu/project/id/242203/reporting
- Feeny D.H., Eckstrom E., Whitlock E.P., et al. (2013) ‘Patient-Reported Outcomes, Health-Related Quality of Life, and Function: An Overview of Measurement Properties.’ A Primer for Systematic Reviewers on the Measurement of Functional Status and Health-Related Quality of Life in Older Adults [Internet]. Rockville (MD): Agency for Healthcare Research and Quality. Retrieved 12 February, 2016, from http://www.ncbi.nlm.nih.gov/books/NBK169156/
- RAND (2016). ’36-Item Short Form Survey from the RAND Medical Outcomes Study.’ RAND Corporation. Retrieved 12 February, 2016, from http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html
- EuroQoL (2016). ‘About EQ-5D’. Retrieved 4 July, 2021, from https://euroqol.org/eq-5d-instruments/
A2-6.07-v1.2