Last update: 18 December 2023
There are currently over 500.000 types of Medical Devices and in vitro diagnostic medical devices (IVDs) on the EU market [1]. Surgical techniques and Medical Devices may seem to have emerged only in the last century, with the development of new products and solutions since the 1990s. However, as early as 2.000 to 5.000 years ago many civilizations used tools such as forceps, knives, scalpels, saws, lancets, needles, trocars, and cautery devices for several medical procedures.
Some of today’s medical devices, such as the tongue depressor, thermometer and stethoscope have been unchanged for centuries, while ‘bionic eyes’ for use in patients with retinitis pigmentosa, a severe eye disease, received regulatory approval for use in humans as recently as 2014. However, rather than discovery in a eureka moment during laboratory exploration, the creation of new medical devices results primarily from clinicians’ ideas and subsequent improvements are often driven by medical device companies. A notable example is the evolution of the electrocardiograph machine (see Figure 1 below).
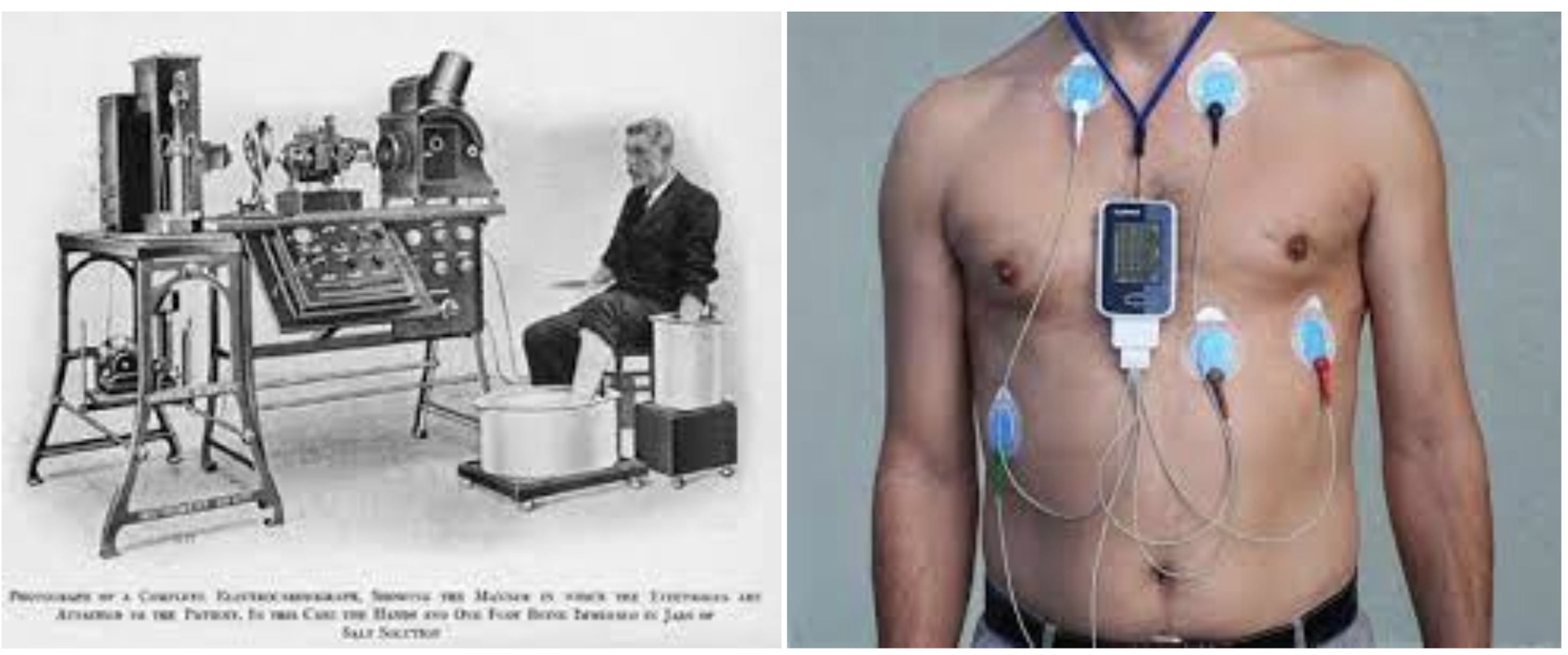
Figure 1: The left image is an example of an early commercial electrocardiograph (ECG). Contrast this with the modern Holter ECG shown on the right. This is a portable device that can remotely relay real-time ECGs to a medical centre while the patient goes about their everyday life (images courtesy of Wikipedia).[2]
What are Medical Devices & In vitro diagnostic Medical Devices?
In general terms, any apparatus, instrument, appliance, software, implant, reagent, material or other article intended by the manufacturers to be used in human beings for specific purposes can be qualified as a‘Medical Device’.
‘In vitro diagnostic Medical Device’ means any Medical Device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for specific purposes.
Phases in the development & lifecycle management of Medical Device
1. Research: Idea – initiation and feasibility phase
2. Design and development (D&D) phase
3. Device production phase
4. Post Market phase
5. Lifecycle management [3]
Global Standards of Medical Device Classification
The classification of Medical Devices under the Medical Devices Regulation (MDR) and In Vitro Diagnostic Medical Devices Regulation (IVDR) is based on the intended purpose of the devices and their inherent risks in a manner that is aligned with many other regions of the world. Thus, the classification of Medical Devices is a risk-based system taking into account the vulnerability of the human body and the potential risks associated with the devices.The higher the risk, the greater the regulatory obligations.
The MDR identifies four classes of Medical Devices, and likewise, the IVDR employs a similar classification system, ranging from Class A (the lowest risk) to Class D (the highest risk) (see Figure 2 below).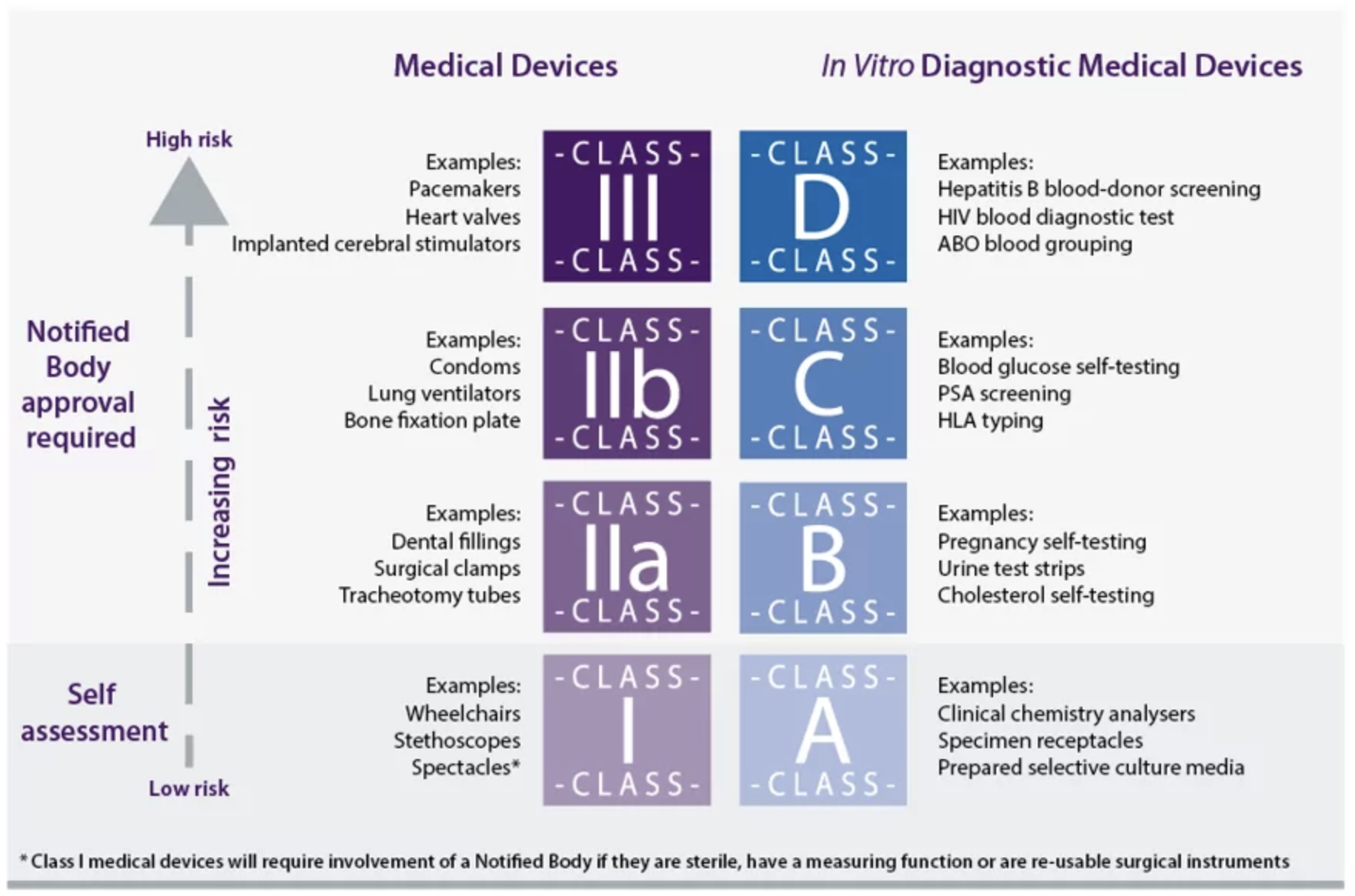
Figure 2: MDR & IVDR Classification System [4]
The risk class determines whether or not a conformity assessment done by a Notified Body (independent certification body) would be required, to ensure Medical Devices are safe and perform as intended [5]. Apart from conformity assessments, clinical evaluation in Medical Devices is also essential to assess the safety and clinical performance of the device in question and to evaluate whether the device is suitable for the purpose(s) and the population(s) for which it is intended [6].
Learning Resources
Take your skills to new heights by earning certification in emerging fields like Medical Devices! Explore the EUPATI Open Classroom and delve into the module ‘Introduction to Medical Devices and their regulatory framework & Medical Devices and Medicine-Device Combinations to deepen your understanding of the Medical Devices’ landscape. Strengthen your expertise, empowering you to advocate for, engage in discussions, and play a pivotal role in implementing key elements that align with your vision for the healthcare system.
References
[1] Overview of the Medical Devices Regulation: https://ec.europa.eu/health/md_sector/overview_en
[2] World Health Organization. “Medical devices: managing the mismatch, 2010.” Available from: http://apps.who.int/iris/bitstream/handle/10665/44407/9789241564045_eng.pdf?sequence=1 (Accessed 3 March 2022).
[3] Steenackers, Wim. “From idea to post-market surveillance: the phases of the medical device lifecycle. Business Development Manager at Quality by design; Medical Devices & IVD; April 26, 2021.
[4] Payne, Sara; Hall, Alison, What is the IVDR?, 2018: https://www.phgfoundation.org/briefing/what-is-the-ivdr
[5] Medical Device Coordination Group (MDCG)
https://ec.europa.eu/health/medical-devices-sector/new-regulations/guidance-mdcg-endorsed-documents-and-other-guidance_en
[6] EU Guidelines on medical devices. Guidelines on clinical investigation: A guide for manufacturers and notified bodies, 2010 MEDDEV 2.7/4


