Last update: 23 November 2015
Introduction
Health Technology Assessment (HTA) is a form of research that generates information about the clinical and cost-effectiveness of health technologies.
In health care, the term health technology (or in short ‘technology’) can include medicinal products (medicines, including biologics), medical devices, equipment, and supplies; medical and surgical procedures; public health programmes and support systems; and organisational and managerial systems used in prevention, screening, diagnosis, treatment, and rehabilitation.
Examples include:
- Medicines
- Programmes to prevent ill-health (e.g. childhood vaccination programmes)
- Procedures (such as surgeries)
- Devices (equipment or machinery that deliver healthcare interventions or assist with activities of daily living, such as an insulin pump or an epinephrine auto-injector)
What does HTA involve?
Health Technology Assessment (HTA) is the systematic evaluation of properties, effects, and/or impacts of health technologies. It may address the direct, intended consequences of technologies as well as their indirect, unintended consequences.
The main purpose of HTA is to inform technology-related policymaking in healthcare, and it is sometimes called ‘the bridge between evidence and policy making’. HTA is conducted by interdisciplinary groups using explicit analytical frameworks drawing from a variety of methods. As HTA bodies and processes vary from country to country (and sometimes region to region) there is no common (harmonised) approach to HTA. However, in recent years the fundamentals of good HTA processes have become more standardised.
The organisational integration of HTA bodies within a healthcare system differs between countries.
Remit
An HTA body must have a defined remit, which will determine what technologies it will or will not assess. The remit of an HTA body also influences many other aspects of the HTA process, including how the HTA body interacts with organisations other than decision-makers, what level of transparency is feasible or required, and the involvement of external people in its processes. The way an HTA body is positioned in a healthcare system plays a key role in the determination of its remit. HTA bodies may exist in different organisations – for example, they may be a part of a health ministry, a quality council, or within a university or a stand-alone legal mandated entity. To be effective, the HTA bodies should have a link with decision makers.
Processes and methods
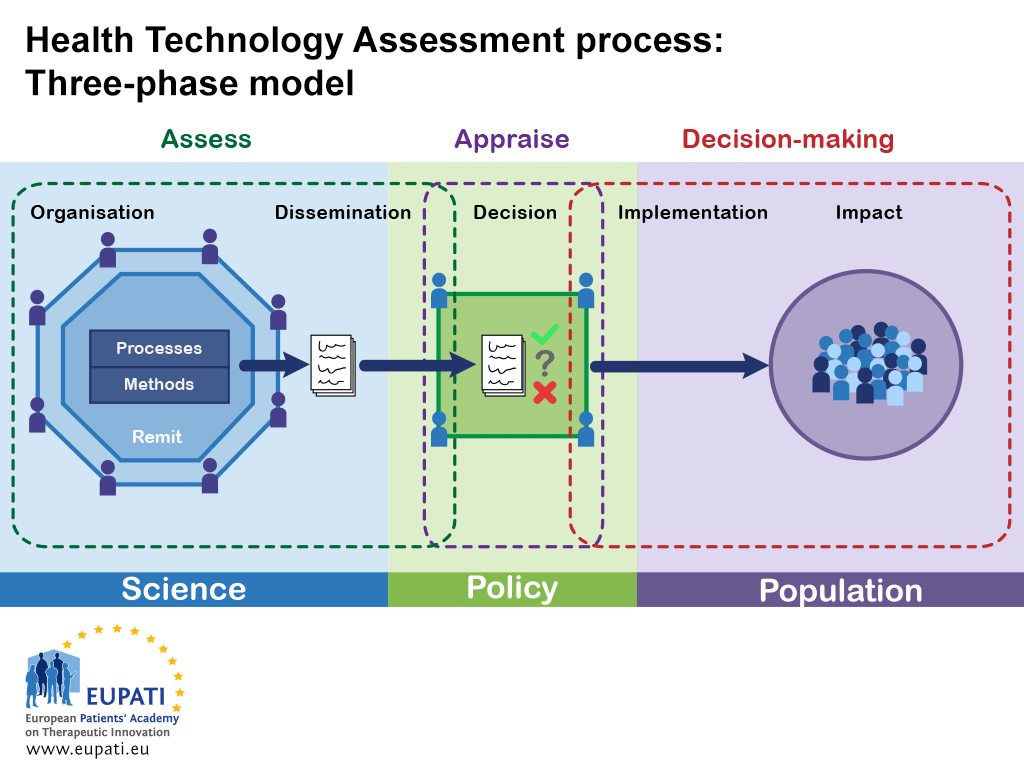
There is great variation in the scope, selection of methods and level of detail in the practice of HTA. In a condensed form, an HTA can be characterised by three phases:
- Assessment: collation and critical review of scientific evidence
- Appraisal: review of the assessment with consideration of all other (policy) factors by a committee to make a recommendation
- Decision-making: implementation of the recommendation
Each of these three phases comprises various structures and functions. Not all HTA programmes conduct all of these steps, in fact ‘decision making’ is rarely, if ever, in the remit of an HTA body.
Figure 1 shows a general (simplified) HTA process. It highlights the goal of the HTA process: communicating information from health research (science) to decision-making and, ultimately, to the population.
- This simplified model of the health technology assessment shows how the three phases interact in the realms of science, policy, and population.
HTA bodies must decide what information is important for decision makers to have about a technology and how they will gather that information. Decisions that an HTA body makes about its processes and methods influence the assessments, and are in turn influenced by the organisation and remit of the HTA body itself.
HTA bodies have to determine what knowledge regarding the consequences and impacts of using a health technology are important for decision-makers and to what extent their assessment should be individualised (to a single technology). For instance, while understanding the clinical effectiveness of a health technology is generally considered important for decision-makers, some health technologies may have ethical issues associated with their use while others do not. An HTA body must choose if it will apply one standard process to all health technologies, or if it will allow for specific processes for the assessment of each technology individually, based on the relevant information required. In this case, should ethical information be gathered for all technologies under evaluation, or should there be a separate process that provides that information where necessary?
Most HTA involves some form of the following basic steps:
- Identify assessment topics
- Specify the assessment problems or questions
- Retrieve available relevant evidence
- Generate or collect new evidence (as appropriate)
- Appraise/interpret quality of the evidence
- Integrate/synthesise evidence
- Formulate findings and recommendations
- Disseminate findings and recommendations
- Monitor impact
Not all HTA bodies conduct all of these steps, and they are not necessarily conducted in a linear manner.
Assessment
The methods and processes used by an HTA body during the assessment phase are important in order to ensure a consistent assessment of new health technologies. If the HTA body fails, for example, to standardise the approach used to conduct a literature review or synthesise the information, they may judge new technologies inconsistently. HTA may use a diverse group of methods.
Two of the main types of HTA methods are primary data collection methods and secondary or integrative methods. Primary data methods involve collection of original data, such as clinical trials and observational studies. Integrative methods, or secondary or synthesis methods, involve combining data or information from existing sources, including from primary data studies. Economic analysis methods can involve one or both of primary data methods and integrative methods.
Many HTA bodies rely largely on integrative methods of reviewing and synthesizing data (using systematic reviews and meta-analyses) based on existing relevant primary data studies (reported in journal articles or from epidemiological or administrative data sets) to formulate findings. Their evaluations are based on distinguishing between stronger and weaker evidence drawn from available primary data studies.
Some assessment efforts involve multiple cycles of retrieving/collecting, interpreting, and integrating evidence before completing an assessment.
It is not always possible to conduct, or base an assessment on, the most rigorous types of studies. Indeed, policies often must be made in the absence, or before completion, of definitive studies. Given their varying assessment orientations, resource constraints and other factors, HTA bodies tend to rely on a combination of different methods.
For instance, to assess a technology, the HTA body may require evidence that addresses multiple issues, including:
- The burden of illness
- Projected epidemiological trends of a disease
- The relative effectiveness of technologies
- The cost-effectiveness of technologies (Note that not all HTA bodies consider cost-effectiveness. Some focus more on added clinical benefit and budget impact.)
- How patients value the outcomes of therapy.
In some cases, an HTA body may answer these questions directly or by commissioning new research. In other cases, they may ask the medicine’s manufacturer to provide the relevant information.
Dissemination
The dissemination of findings and recommendations and monitoring of impact may or may not be part of the HTA itself, but are a separate responsibility of a designated organisation.
In most cases, an HTA report may need to be communicated to people who are not familiar with the details of clinical research or economic evaluation. Depending on the organisation of the HTA body and its relation with decision-makers, the HTA report may need to be conveyed in different formats – such as in print, face-to-face presentation, or both – and may or may not require peer review. How information is presented and the degree of technical language used may depend on whether it is for a committee of experts or directly for a decision-maker.
Decision
Ultimately, the HTA process is intended to support healthcare policy decisions. During this stage of the process, the information gathered and assessed by the HTA body is appraised by decision-makers (for instance, national health systems, insurers, etc.). Although it is assumed decisions are made independently of an HTA body, how decisions are made will have an effect on the HTA process. For example, if decisions can be appealed, then an HTA body may be asked to conduct further analysis. If decisions are made using an expert committee then an HTA body may have to coordinate the committee or attend its meetings. HTA reports may have to be held in confidence or be made available for public comment.
Implementation and impact
When decision-makers have come to a decision based on an HTA report (recommendation), that decision is intended to be implemented within society and consequently will impact on the population. The HTA body’s appraisal process should take into consideration how such decisions are implemented and what the results of their recommendations may be on the population. There may also be a feedback loop in place that allows the HTA body to measure the impact of HTA recommendations on the population in order to take this into account at decision-making stages.
Table 1 below shows the various steps of the HTA process and some relevant questions that an HTA organisation may need to address in order to develop a ‘good’ HTA process. The list of questions is not comprehensive – depending on where the organisation is and who it is supporting, there may be other things to consider.
| Step | Relevant questions for consideration |
|---|---|
| Organisation |
|
| Remit |
|
| Methods and processes |
|
| Dissemination |
|
| Decision |
|
| Implementation |
|
| Impact |
|
Attachment
- Fact Sheet: Steps of HTA processes and their necessary considerations
Size: 119,866 bytes, Format: .docx
This fact sheet provides further considerations for each step of the HTA process.
A2-6.02.1-v1.1