Types of Vaccines
[glossary_exclude]There are vaccines to prevent infectious diseases caused by bacteria (such as pertussis, pneumococcal), viruses (such as measles, mumps, rubella, influenza), and parasites (such as Malaria). Vaccines are also increasingly being developed to treat and not only to prevent. Preventive vaccines fall under four main types:
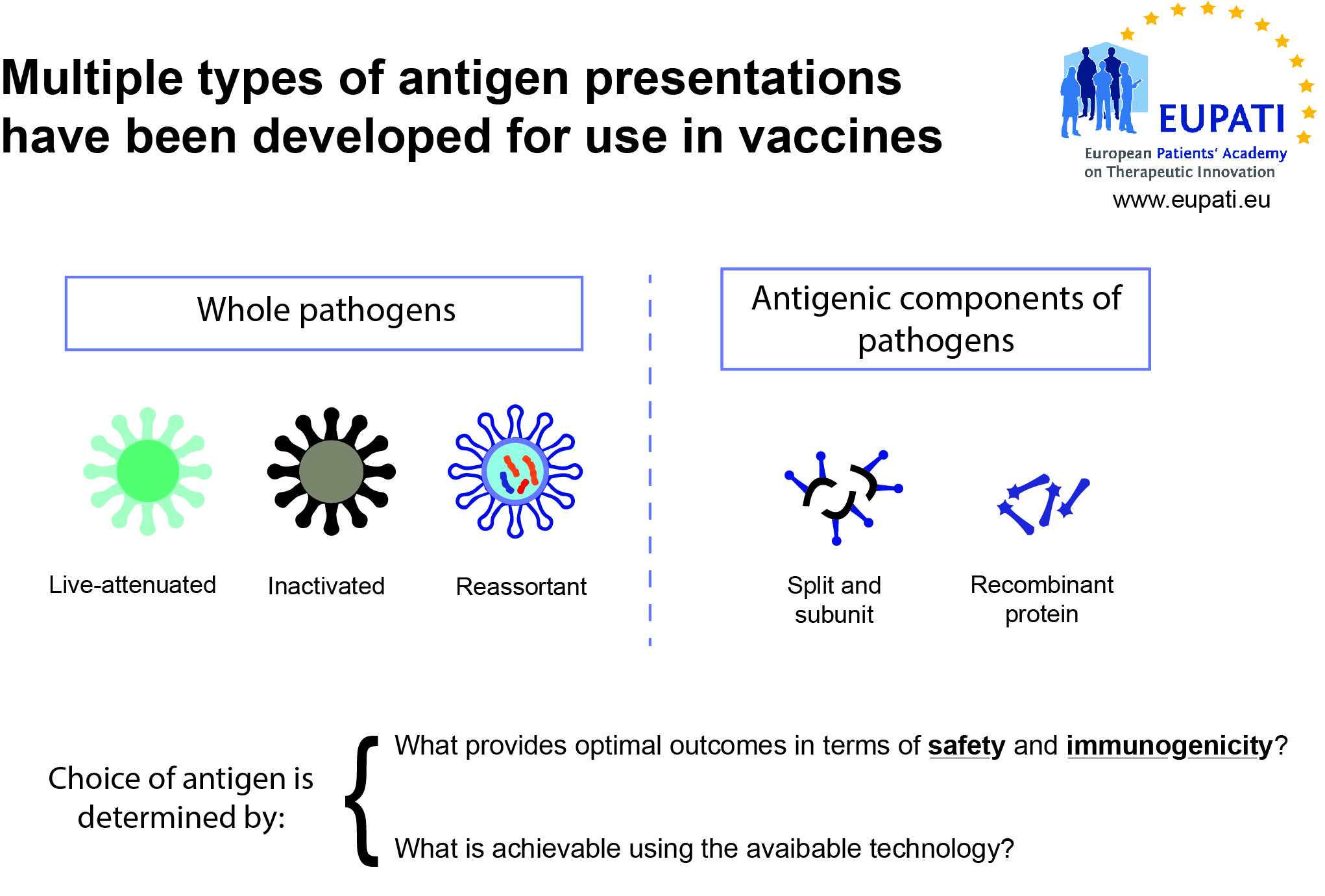
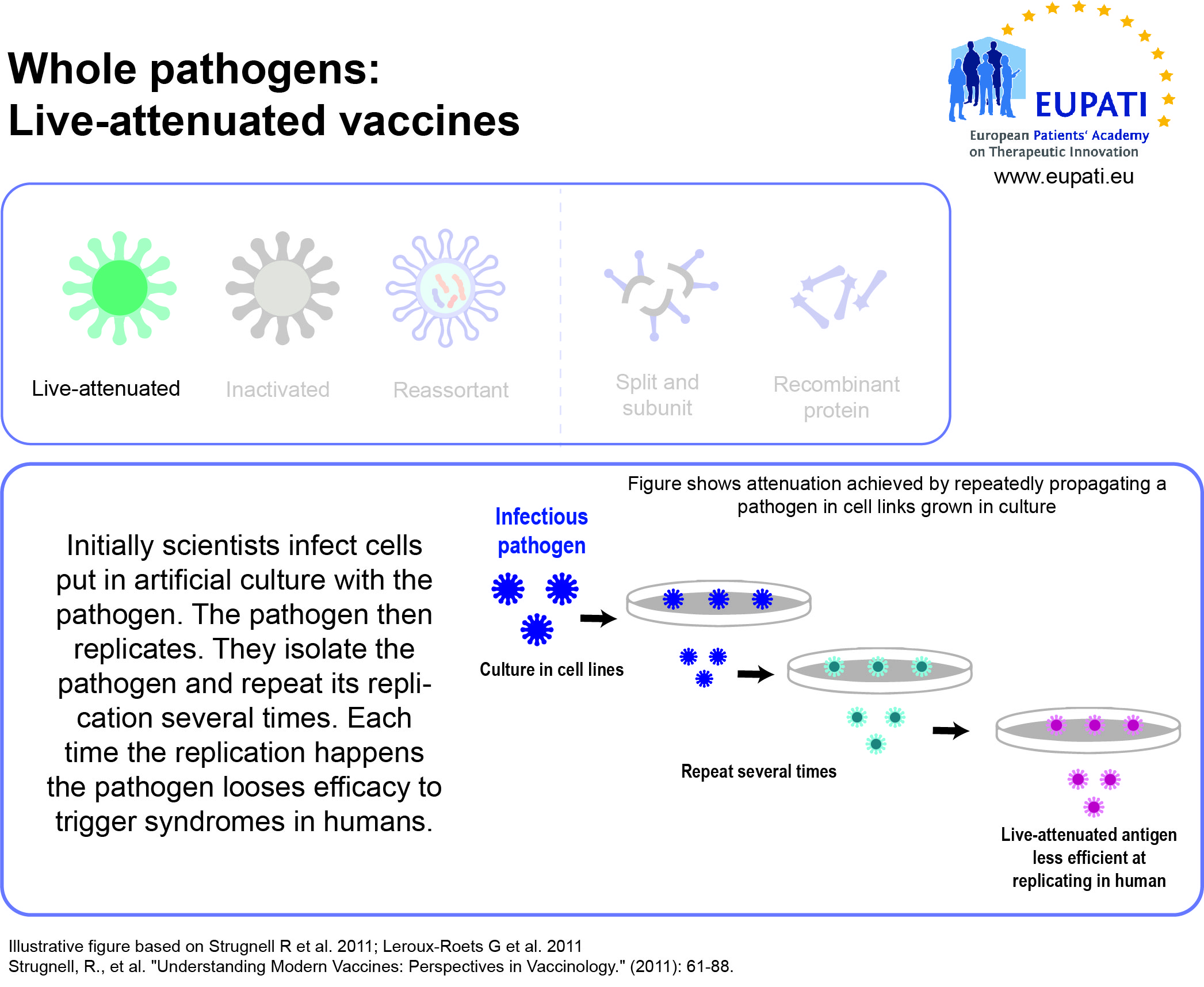
- Live-attenuated vaccines use a weakened (“attenuated”) form of the microbe that causes a disease.
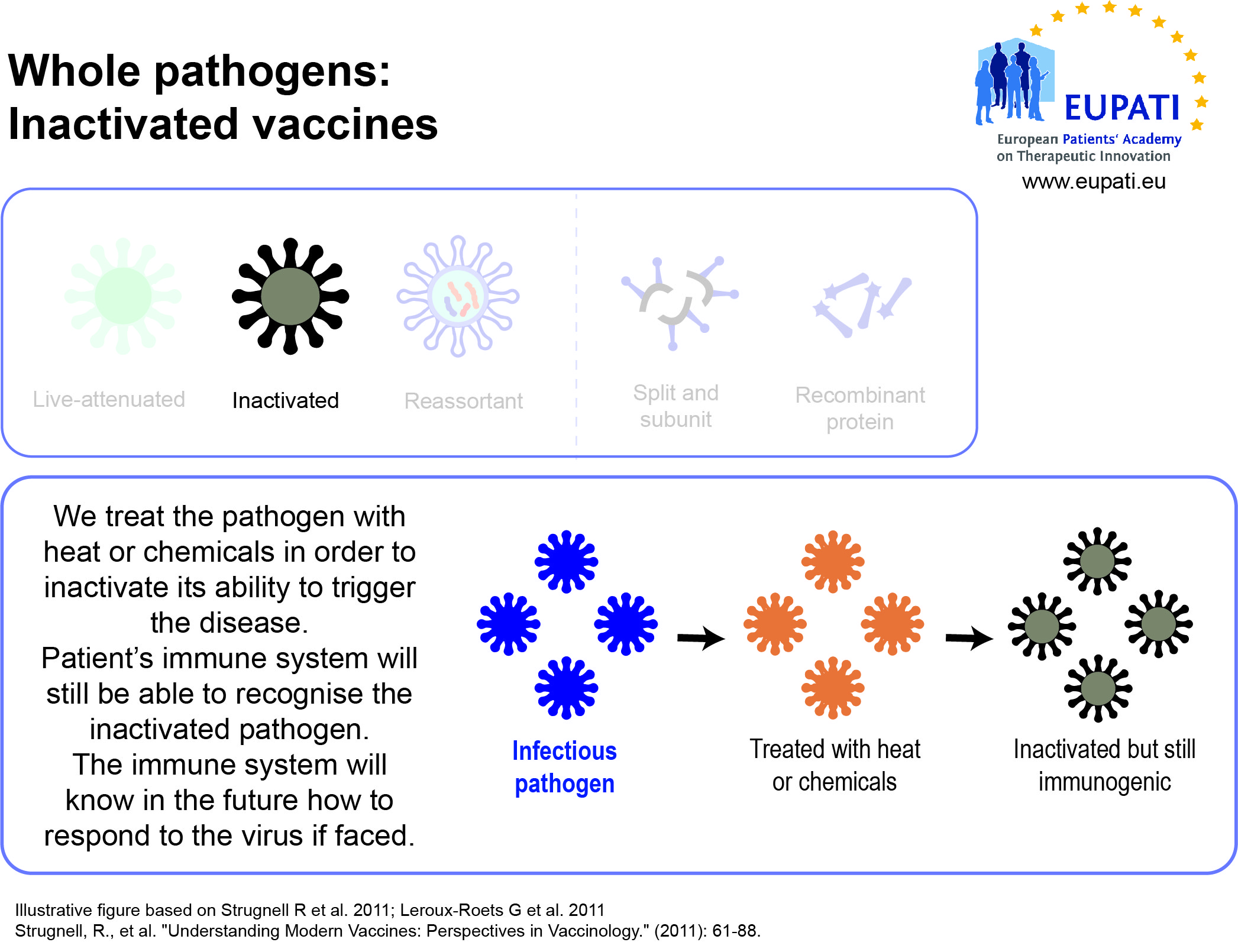
- Inactivated vaccines use the killed version of the pathogen that causes a disease.
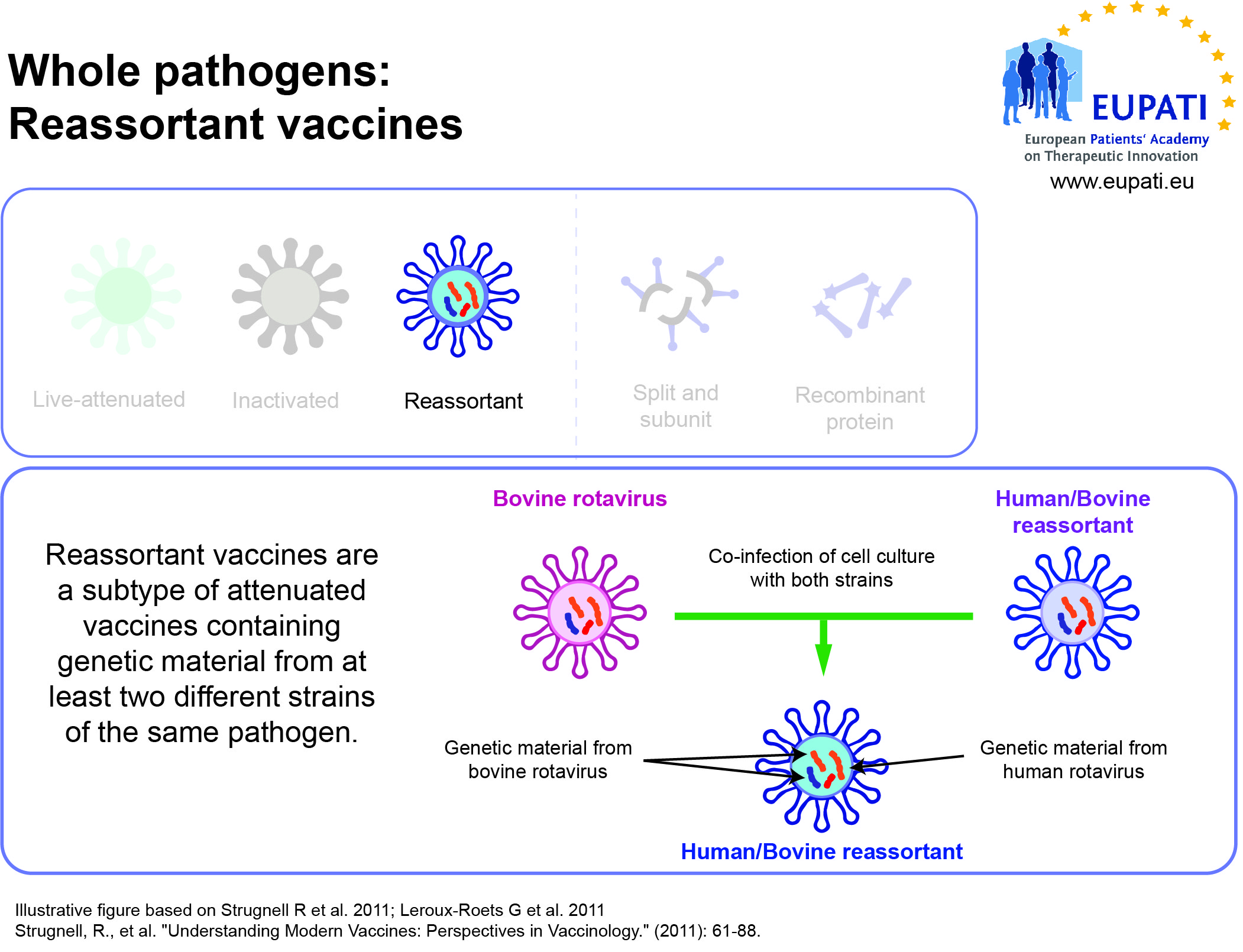
- Reassortant vaccines are made by combining antigens from several viruses or from several strains of the same virus.
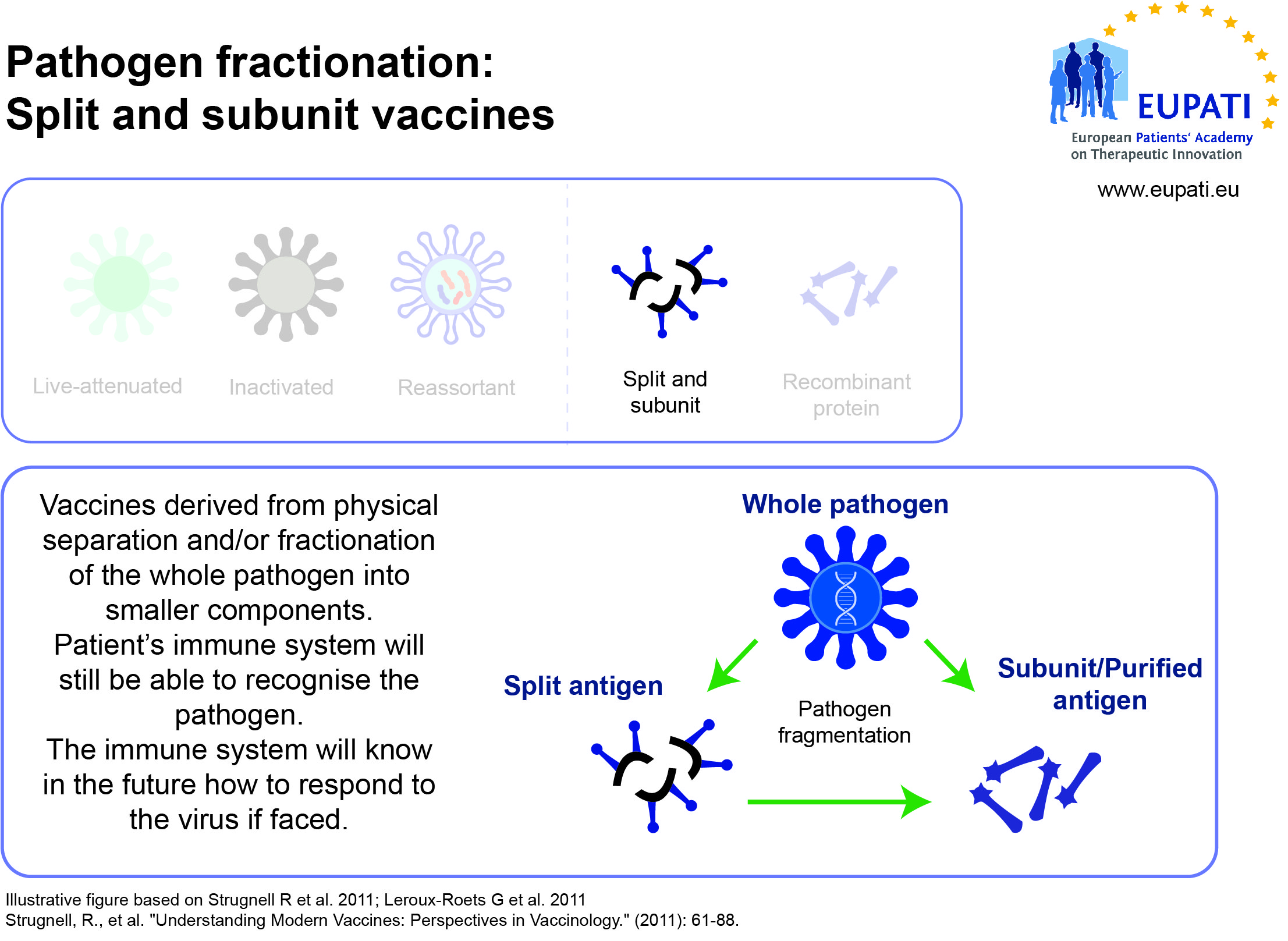
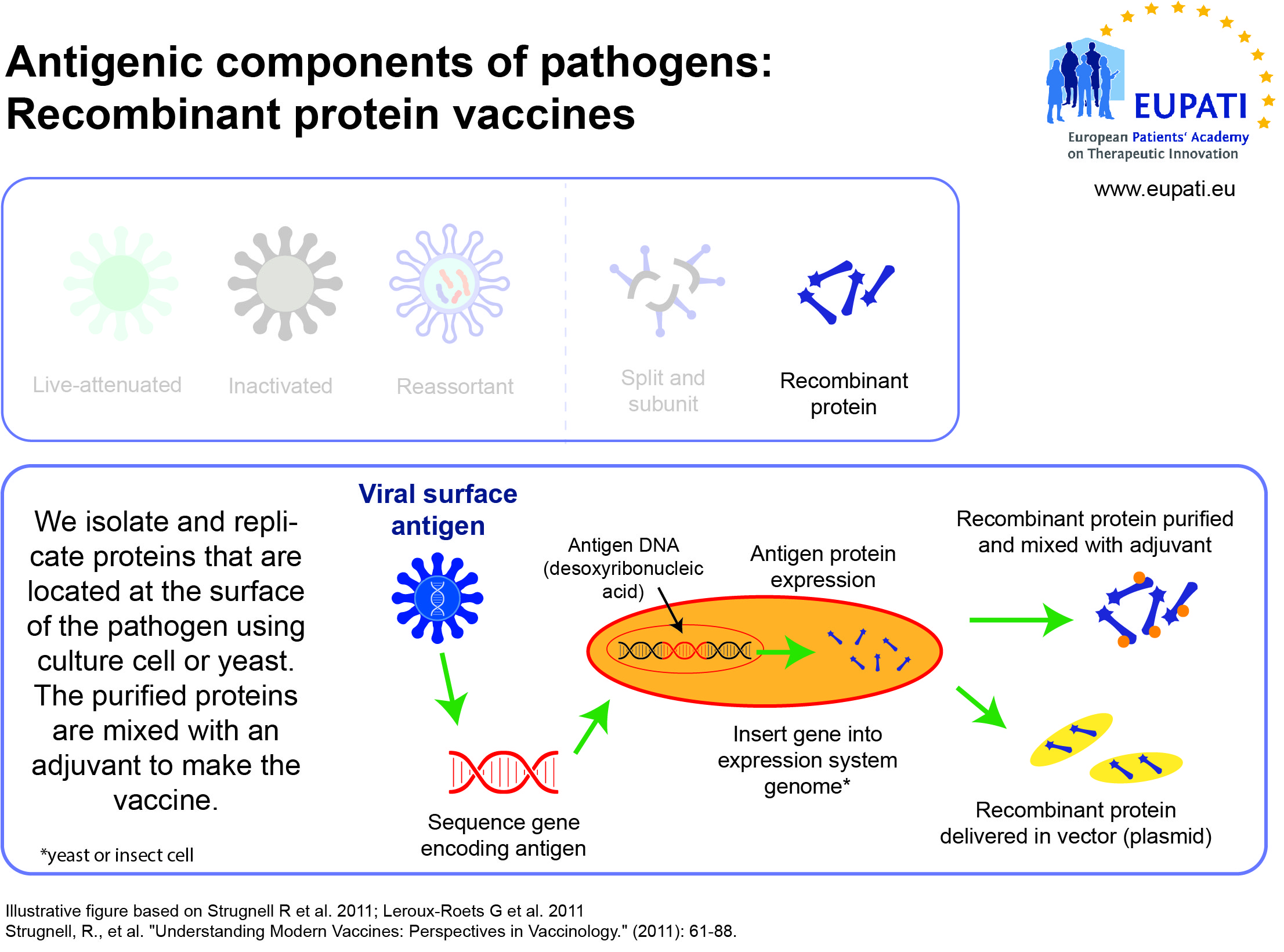
- Subunit, recombinant, polysaccharide, and conjugate vaccines use specific pieces of the pathogen – like its protein, sugar, or capsid (casing around the pathogen).
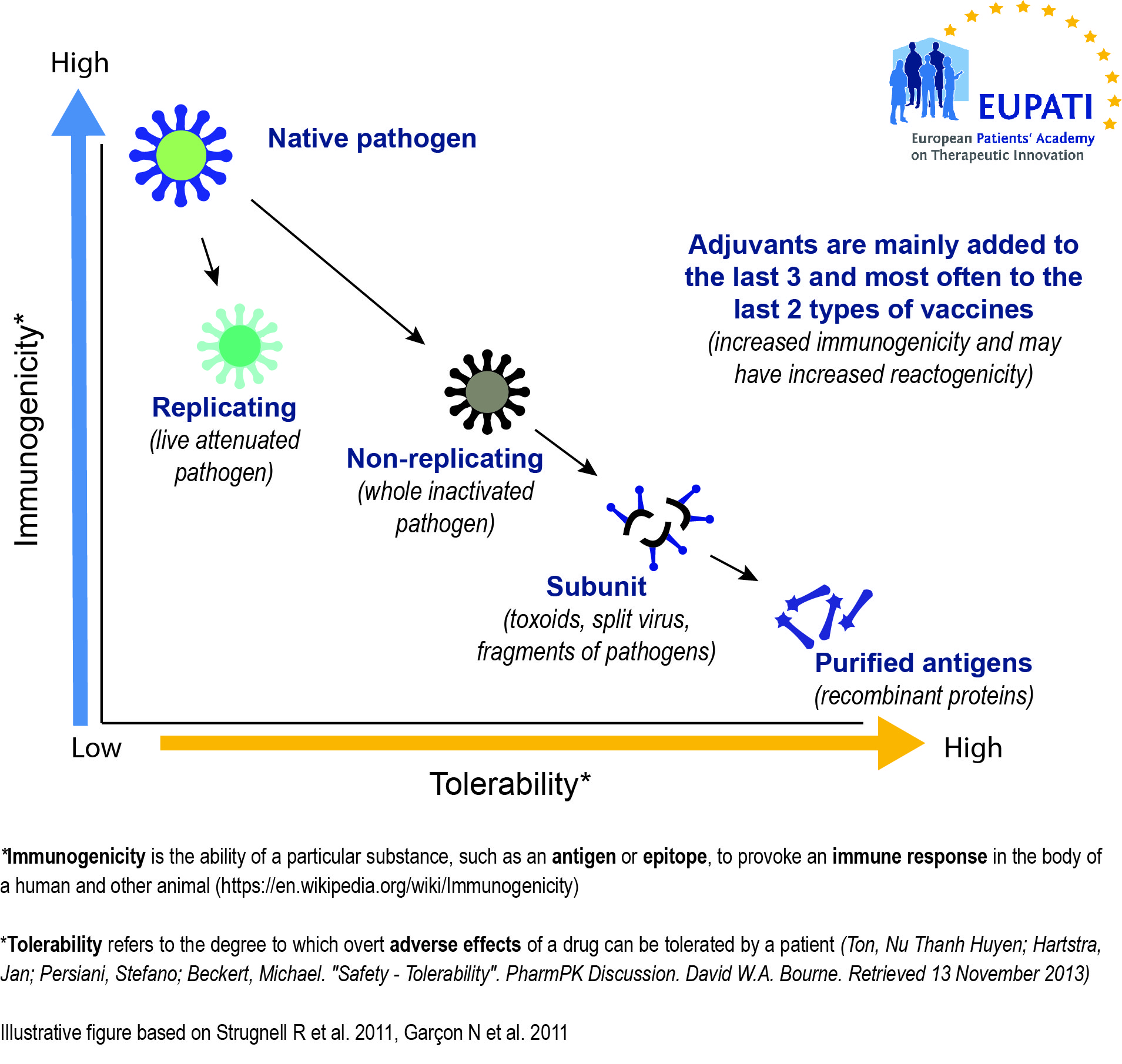
Vaccines need to be efficiently produced and deliverable in a form acceptable to the individual being vaccinated. The earliest vaccines used whole organisms, either alive (attenuated versions of the pathogen, or related but less virulent organisms that could induce cross-reactive immunity without inducing disease) or dead.
Whole organisms have the advantage of being highly immunogenic; they are able to stimulate an immune response similar to that generated by natural infection. Unfortunately, they may also cause reactions similar to the natural infection. A whole-organism vaccine may be ineffective in cases where natural infection does not generate protective immunity. Another limitation is that whole organisms are complex mixtures of proteins, lipids and carbohydrates, which greatly complicates production and quality control of the final vaccine.
Lifecycle of a vaccine
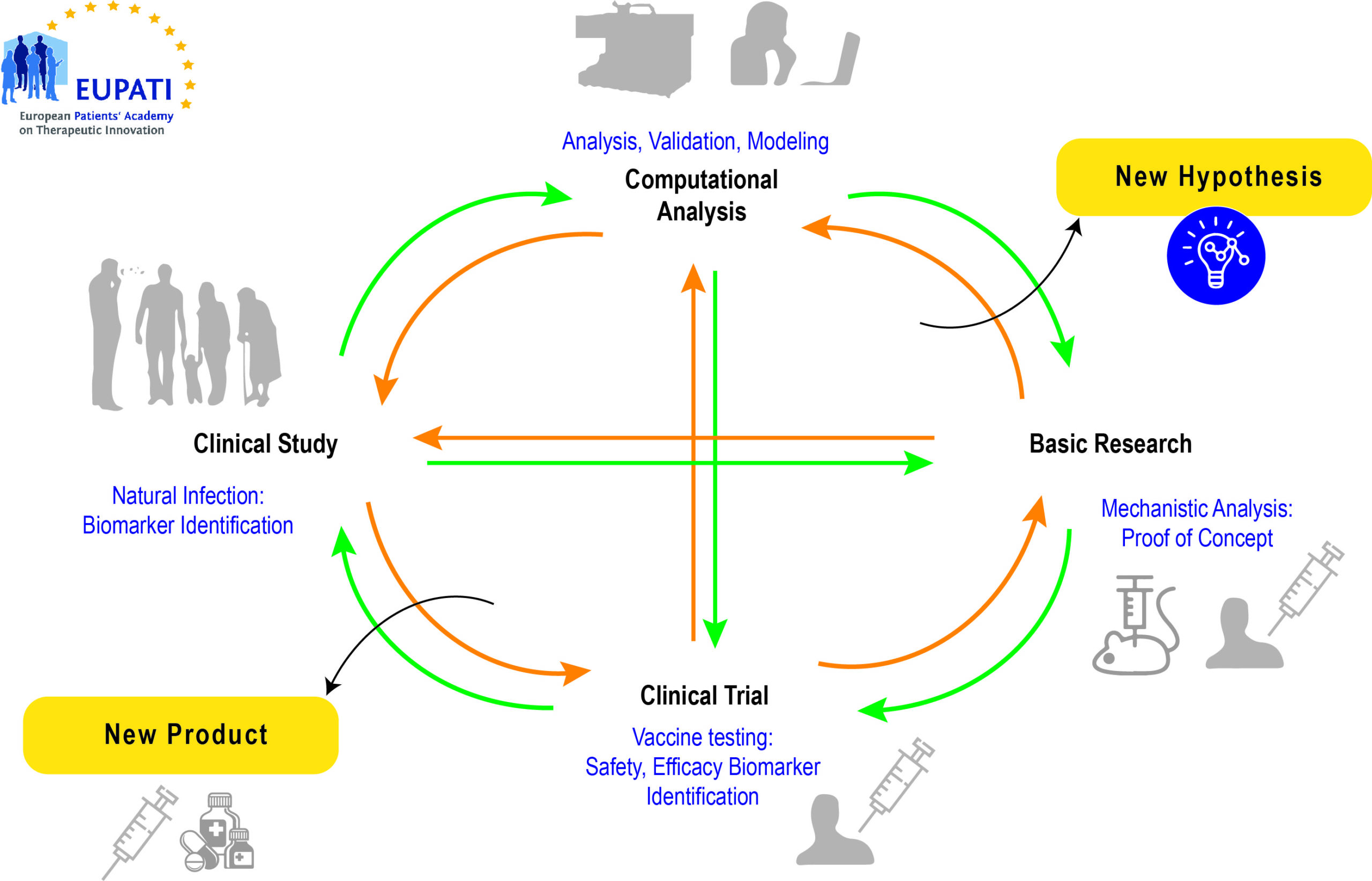
Source: https://www.researchgate.net/figure/Life-cycle-of-modern-vaccine-development-comprising-iteration-of-basic-research_fig1_260374753 (4 July, 2021)[/glossary_exclude]
[glossary_exclude]The importance of vaccination for patients with chronic conditions
More and more diseases may be prevented and/or treated through vaccination. Adults are recommended to receive vaccinations based on their age, underlying medical conditions, lifestyle, prior vaccinations, and other considerations. It is particularly important for patients with chronic diseases to be vaccinated because their immune system is weaker, and they are more likely to develop complications of the condition which may involve long-term illness, hospitalisation, and even death, from certain vaccine-preventable diseases.
For example, according to the European Centre for Disease prevention and Control (ECDC) the analysis of literature indicates that there are two risk groups where routine annual immunisation with seasonal influenza vaccine is justifiable on scientific and public health grounds in Europe.
These are:
- older age groups, usually 65 years and older; and
- people with chronic medical conditions, particularly diseases in the following categories:
- chronic respiratory diseases;
- chronic cardiovascular diseases;
- chronic metabolic disorders;
- chronic renal and hepatic diseases;
- persons with deficient immunity (congenital or acquired);
- young people taking long-term salicylate therapy; and − persons with conditions which compromise respiratory function.
Modes of administration and requirements for formulation
Vaccines are administered in many different ways, such as orally or injection (intramuscular, subcutaneous, intradermal). The choice of mode will depend on a variety of factors, like a pharmaceutical medicine.[/glossary_exclude]
Vaccine Formulation
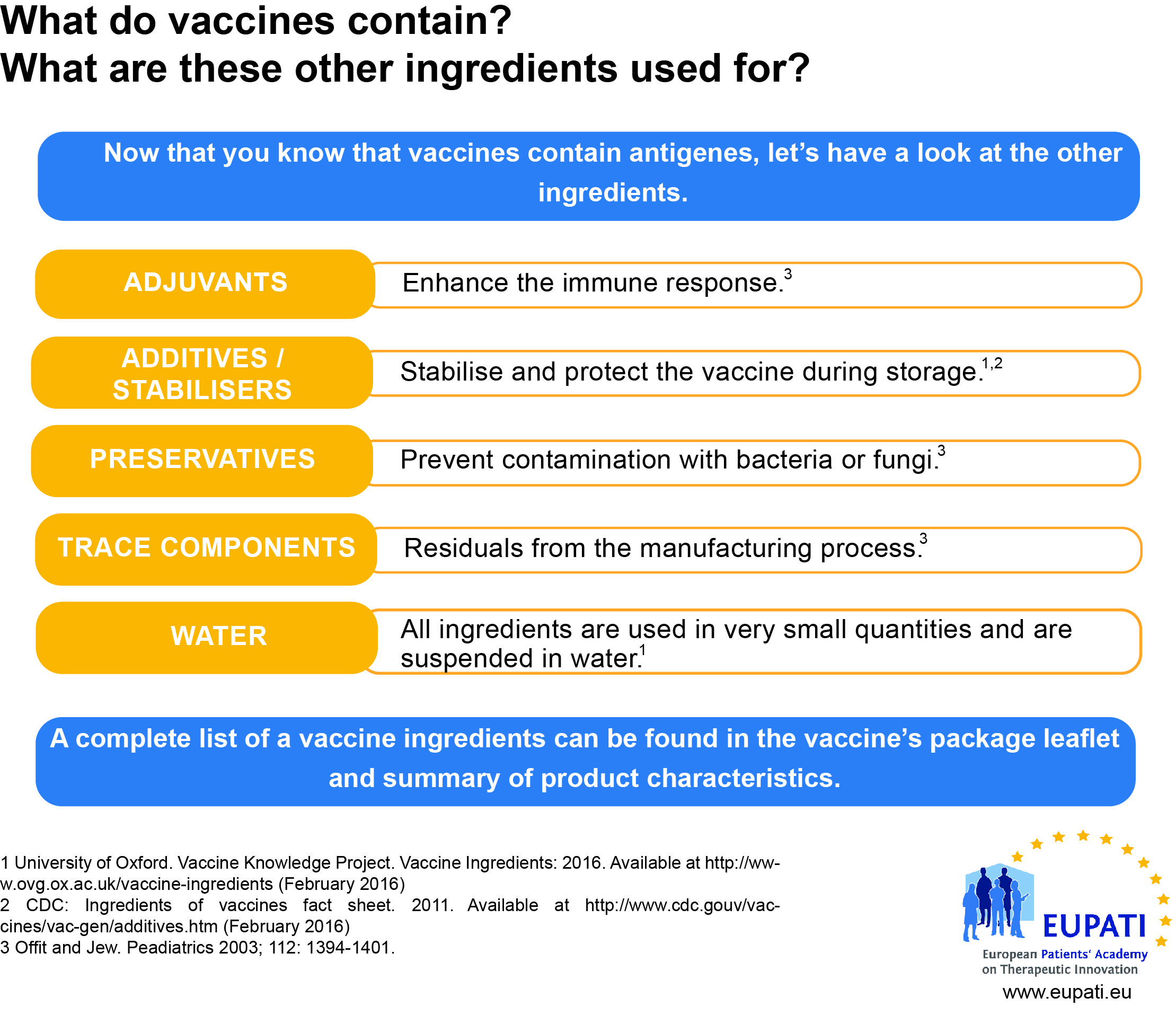
[glossary_exclude]Vaccine Production
Like biological and many new technologies, vaccines are much more complex to produce than traditional small-molecule medicinal products. This requires increased quality control throughout the manufacturing process and supply-chain.
Vaccines are biological substances that may lose their effectiveness quickly or become potentially dangerous if they become too hot or too cold at any time, especially during transport and storage. The ‘cold chain’ is a term used to describe the cold temperature conditions in which certain products need to be kept during storage and distribution.
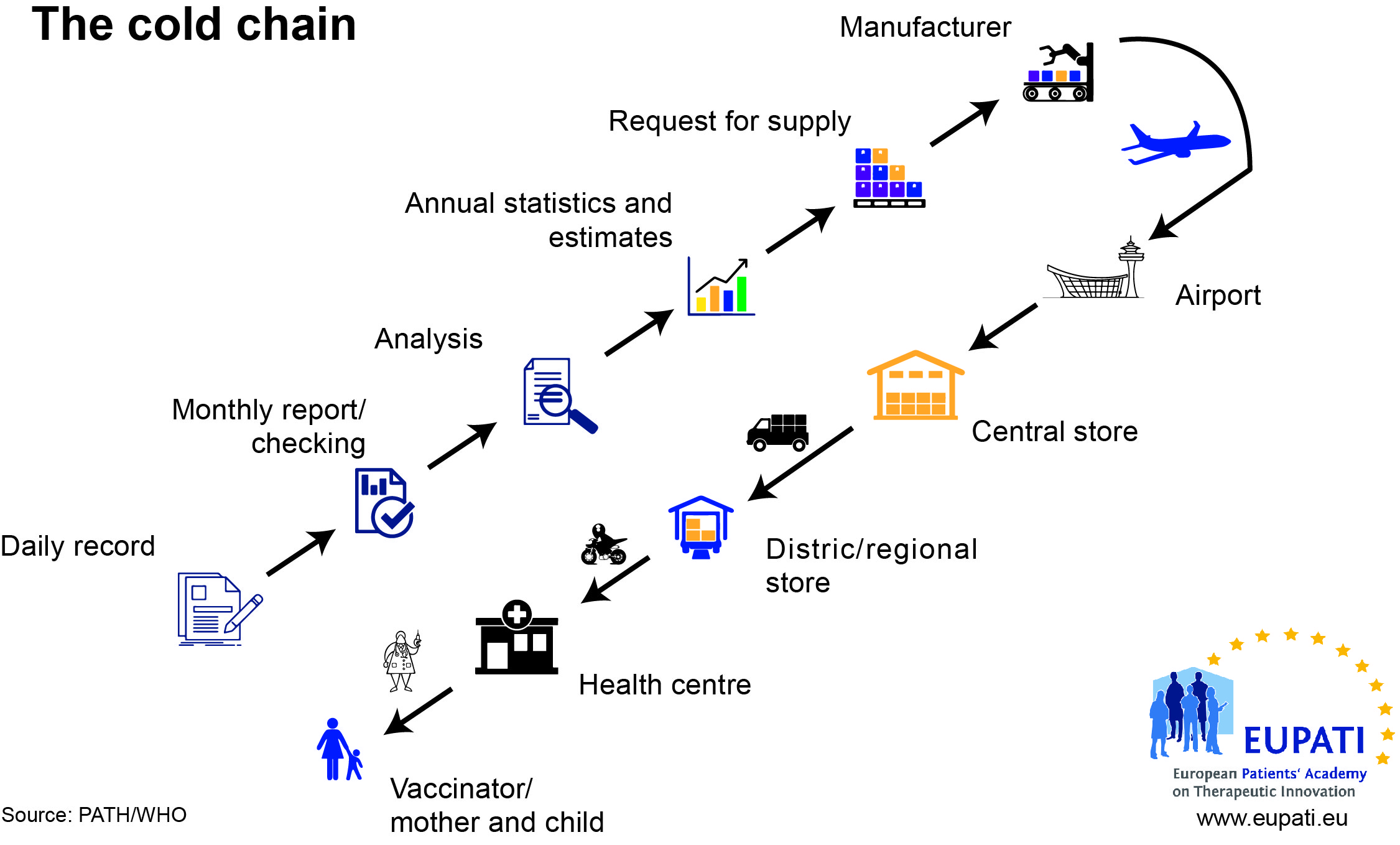
All vaccines must be refrigerated and protected from light. They must not be frozen. The efficacy of vaccines depends on their temperature being kept within the range 2-8°C from manufacturer to patient. Use of a vaccine that has not been stored correctly is likely to be ineffective or even dangerous.
This can prove to be challenging in regions of the world where the environmental temperature varies extremely, and different formulations are needed to cope with these, and reduce the need for a cold chain.
If you would like to read more about the challenges associated with vaccine’s cold chain, please see https://iris.who.int/bitstream/handle/10665/193412/9789241549097_eng.pdf?sequence=1[/glossary_exclude]
[glossary_exclude]Rapid development
In vaccines there are situations which require the rapid development and supply of a vaccine to combat new or recurring . The most common example is seasonal influenza. A new version of the vaccine is developed twice a year, as the influenza virus rapidly changes. This short timeline requires a different approach to the authorisation of a new version of a vaccine, without violating the normal requirements of safety and efficacy. Within the EU, the procedure involves the submission and approval of a core pandemic dossier during the interpandemic period, which is based on a mock-up vaccine. Once a pandemic is declared the procedure allows for fast track approval of a pandemic variation as may be necessary to supplant the vaccine virus in the mock-up vaccine with the final pandemic influenza vaccine virus.
For more information please see https://www.ema.europa.eu/documents/scientific-guideline/guideline-dossier-structure-content-pandemic-influenza-vaccine-marketing-authorisation-application_en.pdf[/glossary_exclude]
[glossary_exclude]Ebola 2014 – A new model for vaccine rapid development?
The March 2014 outbreak of Ebola virus disease in West Africa was the largest and most complex outbreak to date. It affected Guinea, Liberia and Sierra Leone the most and lasted several months.
There was no approved medicines or vaccines to protect people from Ebola or to treat the disease, however, medicines and vaccines against this disease were under development. Some experimental treatments against Ebola have reportedly shown encouraging results in the laboratory or in animals, and even though they have not yet been fully studied in people, there was an urgency to make them available to affected people as soon as possible.
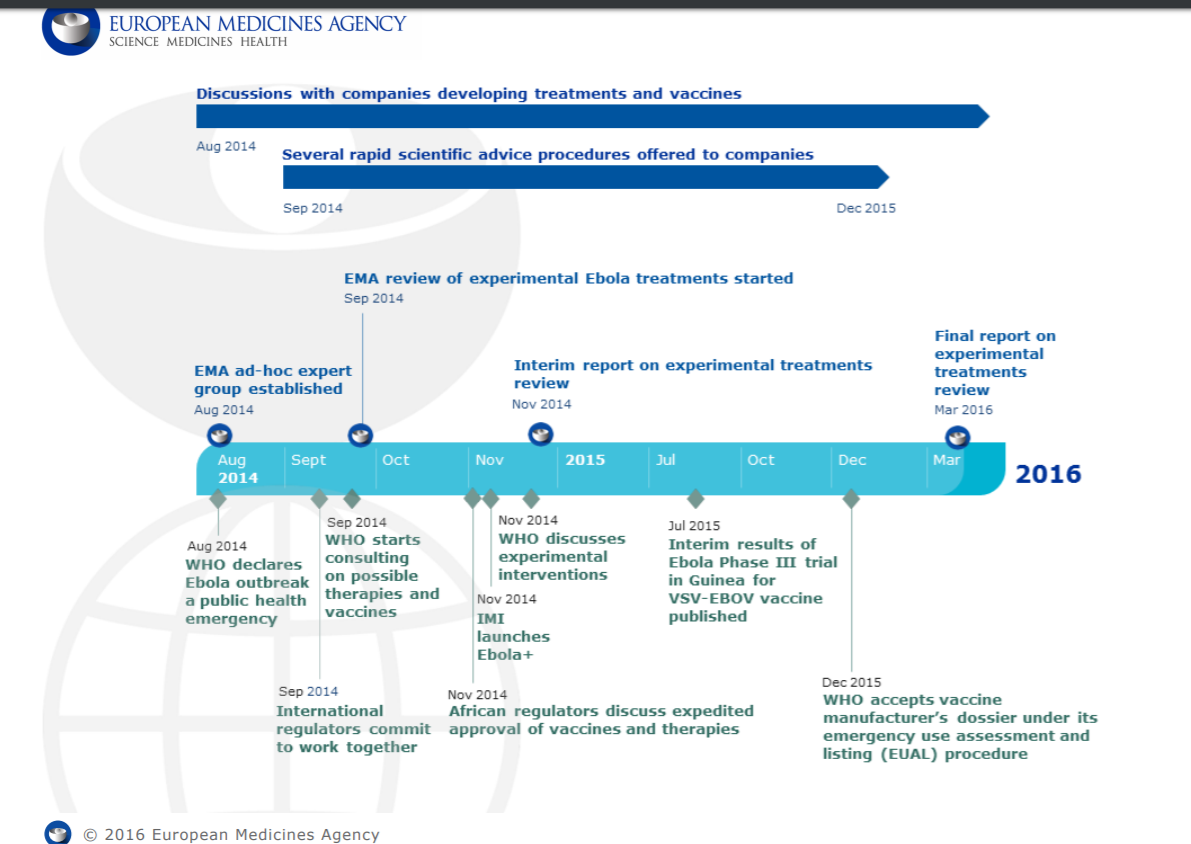 Source: https://www.ema.europa.eu/documents/other/european-medicines-agency-ebola-timeline_en.pdf (4 July, 2021)
Source: https://www.ema.europa.eu/documents/other/european-medicines-agency-ebola-timeline_en.pdf (4 July, 2021)
The 2014 Ebola outbreak triggered intensive efforts on a global scale involving collaboration between manufacturers, regulatory authorities, and WHO to pioneer new processes to allow for the rapid development of a new vaccine. Should future pandemics occur, this model can be used to ensure a fast response.[/glossary_exclude]
[glossary_exclude]Pharmacovigilance
Like a medicine, vaccines undergo pharmacovigilance activities. Read about Pharmacovigilance here.[/glossary_exclude]
Patient Involvement in vaccine development
Since the vaccines development process is comparable to the development process of medicinal products, there are similar opportunities for patient involvement throughout the development cycles. Differences are seen in early development, where there is a need in medicines development to trigger development by being involved in the definition of research priorities. Conversely in vaccines, development is often triggered by public health needs. Community involvement is particularly challenging in preventative vaccines development, as there are usually no organised groups of potential patients to involve. For example, the primary target group to consider for routine vaccination against the Human Papilloma Virus (HPV) is girls at the age just before sexual activity.
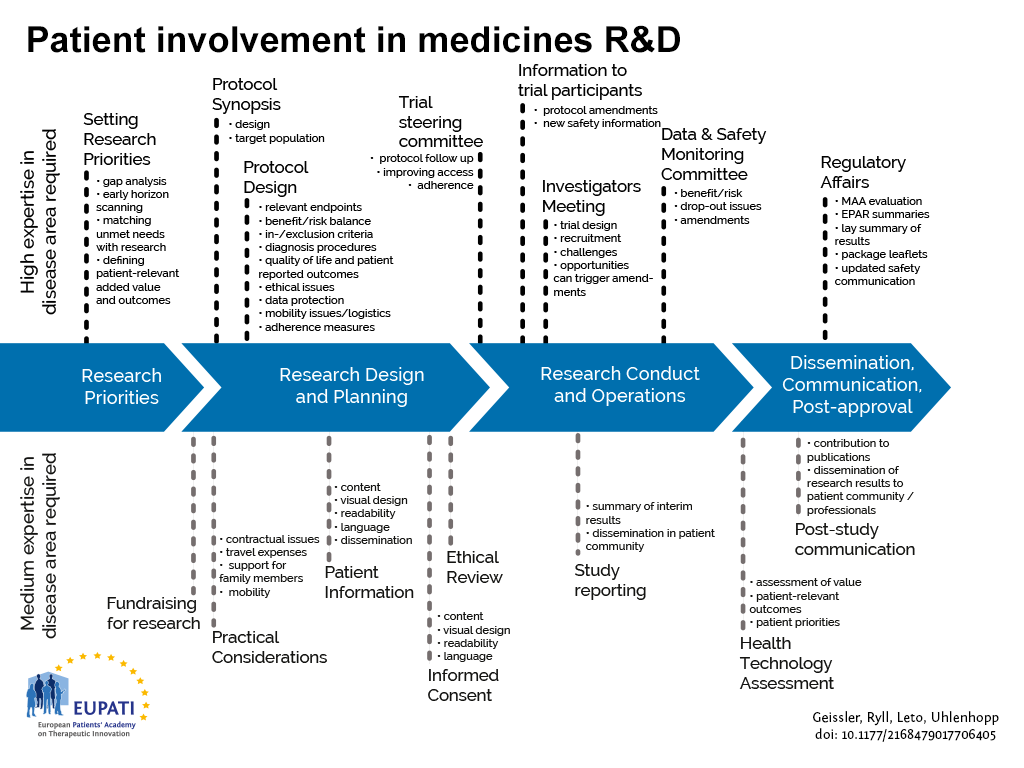
Currently most opportunities for patient involvement are concentrated in the clinical development phases.
[glossary_exclude]Vaccines and health policy
If you are interested in the vaccine health policy debate you can find information from your local authorities and health ministries, or on a regional and global level from organisations such as:
- World Health Organisation – https://www.who.int/topics/vaccines/en/
- European Patients’ Forum – https://www.eu-patient.eu/policy/Policy/vaccination/[/glossary_exclude]
[glossary_exclude]References
- 1https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/223753/Green_Book_Chapter_3_v3_0W.pdf
- http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_120010
- https://www.ema.europa.eu/documents/scientific-guideline/guideline-dossier-structure-content-pandemic-influenza-vaccine-marketing-authorisation-application_en.pdf
- https://www.ema.europa.eu/documents/other/european-medicines-agency-ebola-timeline_en.pdf[/glossary_exclude]