Translational medicine
1. Introduction
Translational medicine also referred to as translational science or translational research, is defined[¹] as an area of biomedical research that aims to improve human health and longevity by determining the relevance to human disease of novel discoveries in the biological sciences. It is a rapidly growing discipline that is multidisciplinary in nature and links observations of adjacent fields with the aim to create novel or improved preventions, diagnoses, and treatments of disease. The translational process involves close collaboration of many stakeholders such as academia, industry, patients, regulators and the public to address unmet medical needs. Translational medicine does not only cover the underlying research and scientific disciplines, but also considers the operational and regulatory aspects to ensure optimal planning, robust and reliable results and address systemic bottlenecks which slow down bringing innovations to patients.
The field aimed at optimising the efficiency of the translational process is defined as translational science and focuses on understanding the scientific and operational principles underlying each step of the translational process (see section 2.4).
Translational medicine is often described as the practice of transferring scientific knowledge “from bench to bedside“ and “from bedside to bench”(B2B) (see below, section 2.2). Translational medicine builds on basic research advances – including studies of biological processes, for example, using cell cultures or animal models – and uses fundamentally new insights obtained with these models and integrates these with clinical observations to develop new therapies or medical procedures.
2.1 Core concept and history of translational medicine
Translational medicine converts promising laboratory discoveries into clinical applications and addresses clinical questions to facilitate prediction, prevention, diagnosis, and treatment of diseases. Translational research takes academic basic research findings further into applied research where, in the ideal case, there is a continuum between the research in academia and development in partnership with other stakeholders with a clear end-goal for the patient in mind. End-goals can be defined as improved treatment regimens, novel therapeutics or diagnostics with clearly defined outcome measures, such as improved quality of life. Thus, translational medicine transforms the basic research achievements of medical biology into practice, with new technologies, and methods that will bridge laboratory and clinical practice.
Involving the patient as a key stakeholder actively in translational medicine processes ensures novel strategies are addressing the unmet needs of the patient population, create concrete solutions designed accordingly – based on understanding what matters to patients in real life conditions, what could provide them a better quality of life etc.
History of translational medicine
The term translational medicine was introduced¹ in the 1990s, but only gained wide usage in the early 2000s. Originally, translational medical research emerged from the concept of bench-to-bedside (B2B), as a class of medical research aiming to eliminate the barriers between laboratory and clinical research, acting in their siloes. Translational medicine developed as an overarching discipline from cross-talk between several scientific disciplines such as clinical pharmacology, cell biology, genetics, chemistry, physiology etc. The maturation of translational medicine into a recognised discipline itself also required the introduction of non-scientific, process-oriented aspects and the contributions from the several disciplines, breaking boundaries among them and bringing them together in a concerted action to streamline translation of research discoveries into concrete solutions for patients.
In 2003, the Institute of Medicine Clinical Research Roundtable described² the current terminology and model of translational research as a two-phase process of research, progressing from:
- Basic science to clinical science
- Clinical science to public health impact
Further refinement in the following years led to the translation model postulated as the 4 T’s model:
- T1: basic scientific discovery (basic knowledge) to potential clinical application (theoretical knowledge) to
- T2: evidence-based guidelines (efficacy knowledge) to
- T3: clinical care or intervention (applied knowledge focusing on implementation) to
- T4: the health of a community or population (public health knowledge on effectiveness)
After a systematic literature review and analysis² in 2017, an extra phase T0 was proposed that involves such as genome-wide association studies, which wrap back around to basic research (T1).
Rather than viewing these 4Ts as different (isolated) phases, from an operational perspective, the field of translational science considers translational medicine as a spectrum, or continuum.
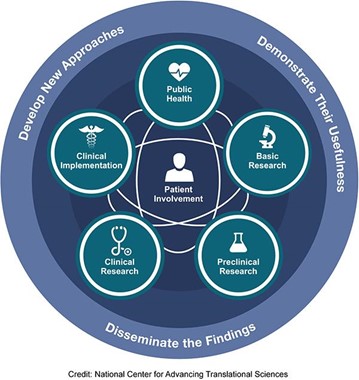
According to the NIH National Academy of Advanced Translational Sciences (NCATS)⁴, each stage of research is represented along the path from the biological basis of health and disease to interventions that improve the health of individuals and the public. The spectrum is not linear or unidirectional; each stage builds upon and informs the others. All steps of the spectrum require development of new approaches, demonstration of their usefulness and dissemination of the findings. Patient involvement is a critical feature along the continuum of translational medicine.
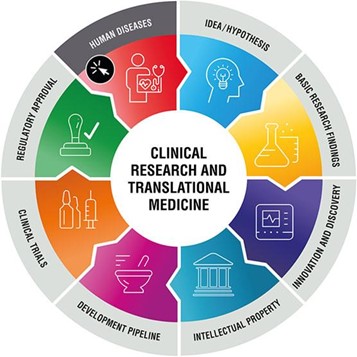
According to Monash University⁵, as shown in the following figures, clinical research and translational medicine take a central place in connecting the various disciplines in this spectrum or continuum.
Figure (1): The continuum of translational medicine
Graph source: https://www.monash.edu/medicine/ccs/translational
Figure (2): The continuum of translational medicine
Graph source: https://ncats.nih.gov/translation/spectrum
2.2 Translational medicine: a bi-directional approach
Translational medicine will encourage the flow of information and new clinical research hypotheses from the laboratory to the clinic („from bench to bedside”), and in the same way, it encourages translation of observations from the clinic back to the laboratory („from bedside to bench”). This means that translational medicine, as a concept, is a bi-directional concept, encompassing:
- bench-to-bedside factors, which aim to increase the efficiency of testing new therapeutic strategies developed through research clinically, and
- bedside-to-bench factors, which provide feedback about the applications of new treatments, diagnostics and preventive measures, where clinical observations can inform research to generate new hypotheses. Through the use of real-world evidence or real-world data, these applications can be improved and generate new solutions. For example, the development of auto-antibodies in patients with certain diseases, could form a basis for new methods of patient stratification and development of new or better treatments.⁶
Currently, advances in the understanding of biologic systems and the development of novel translational research tools that can be applied at both the bench and the bedside offer unprecedented prospects for advancing knowledge of human disorders in this bi-directional translational context.
Translational medicine seeks to coordinate the use of new knowledge in clinical practice and to incorporate clinical observations and questions into scientific hypotheses in the laboratory. It also facilitates the characterisation of disease processes and the generation of novel hypotheses based on direct human observation. More and more, patients and patient organisations play a very important role in ensuring continuous feedback and communication to the scientific community, as part of the multistakeholder approach (see section 2.3) that is an essential factor for success in the process of the translation process.
The ultimate goal of translational medicine is to help patients, with a more rapid development of new diagnostics, medicinal products and new medical knowledge for treatment and prevention of diseases, enabling access to care for people at reasonable costs.
2.3 A Multi-stakeholder approach
The continuum of translational medicine thrives on an efficient interplay of many disciplines, that spans chemists, biologists, data scientists in research in academia, clinicians and healthcare specialists from academic medical centres, technology experts from core research facilities, regulatory agencies like the European Medicines Agency (EMA), the Food and Drug Association (FDA) in the US and the national competent authorities in each EU member state, pharmaceutical and product development experts from industry, legal and ethical experts, patients as an individual or represented as a group by organisations connecting to society, research funders and policy makers. Governments may play an important role in creating a favourable ecosystem, providing critical infrastructure, incentives and addressing cross-sectoral barriers. The success of translational medicine is ultimately defined by how the system can adopt innovation with acceptable cost-benefit ratios. Health economics experts can guide this process so that health insurers will provide reimbursement of novel cost-effective therapies, diagnostics and interventions towards practical implementation in the healthcare system. Once launched, the post-market surveillance studies (real-world data or real-world evidence) can collect data that can flow back into research (“bedside to bench”).
Due to the involvement of different stakeholders and disciplines, there is no one definition of translational medicine, but different perspectives play a role on how translational medicine is described by a particular stakeholder group.
For those who are interested, the following list highlights some of the different aspects:
- Barry S. Coller from the Rockefeller University, NY defines translational medicine as: “The application of the scientific method to address a health need.” He holds that, in contrast to basic research, which has the generation of new knowledge as its primary goal, the primary goal of translational science is improvement in human health.*
- John Hutton from the Cincinnati Children’s Hospital Medical Centre, described translational medicine as follows: “Translational medicine transforms scientific discoveries arising from laboratory, clinical or population studies into new clinical tools and applications that improve human health by reducing disease incidence, morbidity and mortality” and improve the quality of life. This definition has been taken and adapted from “Transforming Translation – Harnessing Discovery for Patient and Public Benefit” (Report of the Translational Research Working Group of the National Cancer Advisory Board, US NIH, 2007).*
- Elias Zerhouni (former NIH director, former Sanofi CEO, one of the founders and advocates of modern translational medicine) stated that the system is fragmented without alignment of all stakeholders leading to duplication of efforts and misalignment with the healthcare system and patient needs. Increasingly complex resources needed to conduct modern clinical and translational research are missing or scattered and investment in methodologic research to improve the tools used by clinical and translational scientists is key.⁷
- Chris Austin (NCATS) defines translation as the process of turning observations in the laboratory, clinic and community into interventions that improve the health of individuals and the public -ranging from therapeutics and diagnostics to medical procedures and includes behavioural medicine. More specifically, the field of translational science is the investigation focused on understanding the scientific and operational principles underlying each step of the translational process.*
- EATRIS, (the European infrastructure for Translational Medicine) has a vision to make the translation of scientific discoveries into medical products more effective to improve human health and quality of life. Its mission is to support researchers in developing their biomedical discoveries into novel translational tools and interventions for better health outcomes for society. In this European research consortium, capacity is brought together with centralised access to academic and clinical research facilities and translational research models with the aim to turn laboratory findings more efficiently into solutions for the patient.
- Eric Topol (head of Translational Research at Scripps institute) emphasises the individual aspects of the patient in the translational medicine process (personalised medicine), in that “we need to be far more clever and much more elegant about biologically exciting ways to match up the patient at risk — the biology of the individual—[with] the particular intervention or therapy.”⁸,⁹
- Merck has developed a translational medicine guide¹⁰ for their internal drug development process that recognises that translational science can disruptively improve clinical development and product success, but that it requires changes to mindset as well as governance structures.
- Novartis¹¹ has a dedicated department that develops biomarkers and assays to investigate whether a given therapy alters the same disease pathways in people as it does in preclinical studies in model organisms. By using imaging data, genetic expression profiles and other powerful tools, they are able to explore treatment responses in far more sophisticated ways than was possible in the past. In that way, they let the science point them towards the best medical applications for a new therapy, instead of committing to just one disease area early on.
- The above view is recognised by the American Society for Clinical Pharmacology & Therapeutics (ASCPT)¹², that also holds a broad definition of translational medicine. Translational research in clinical pharmacology may include evaluation of various biomarkers of pharmacologic response and assessing the linkage between biomarker response and clinical endpoints in patients. And that includes how the response to a therapeutic intervention in a particular disease may translate to a response in another disease, as well as translation of safety signals across species and/or patient populations. Translational research is bolstered by quantitative, model-based and mechanistic understanding of disease biology and pharmacology. Consequently, core disciplines, including clinical pharmacology, pharmacogenomics, systems pharmacology, precision medicine, as well as others play an integral role in enabling translational research and translational medicine.
- “Science Translational Medicine”, a leading journal in this field, publishes “original, peer-reviewed, science-based research articles that report successful advances toward the goal of improving patients' lives”, highlighting its very wide scope and audience.¹³
To make translational medicine work requires all these stakeholders with different perspectives to work in concert. That sometimes requires novel, non-conventional collaboration models putting emphasis on the sharing of common goals, with the patients’ needs as the overarching goal. Each actor having their own part to play: the academic knowledge generator, educator and researcher generating and testing new hypotheses, the industry innovator performing applied research, validation of results and product development, the business developer creating a viable business case substantiated by marked needs and exploiting intellectual property, and the patient as a co-creator by informing on the patient’s needs identified and confirming the relevant clinical endpoints and product characteristics as the overarching goal. These actors are involved in an intricate play of translation where collaboration between disciplines is key.
Examples of organisations involved in the field of translational medicine, performing translational science, translational research and support in its organisation are the NIH National Center for Advancing Translational Sciences NCATS (established in 2012)¹⁴, the European Infrastructure for Translational Medicine EATRIS (established in 2014)¹⁵, the European Alliance of Biomedical Research Infrastructures AMRI (established in 2021)¹⁶, and the Innovative Medicines Initiative IMI¹⁷. IMI1 ran from 2008-2013, IMI2 until 2020 and it will continue as the Innovative Health Initiative¹⁸ from 2021 onwards. On the global stage, Translation Together¹⁹ was launched as a collaboration between NCATS (US)²⁰, EATRIS (Europe), LifeArc (UK)²¹, Admare (Canada)²², TIA (Australia)²³, FIOCRUZ (Brasil)²⁴ and AMED (Japan)²⁵.
2.4 Translational Science
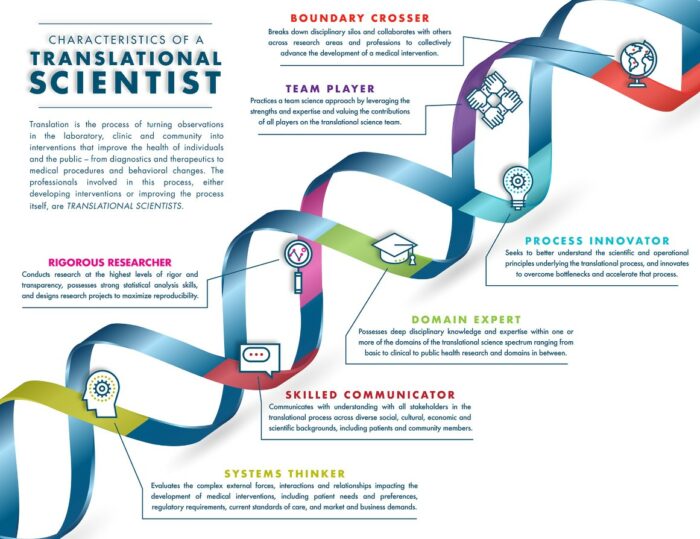
An important aspect of translation is, beyond the scientific elements, the specific attention to the non-scientific, i.e., operational, regulatory, organisational, cross-sectoral and multidisciplinary elements of the process. Implementation of these non-scientific aspects in translational medicine enables a more efficient process of translation of biomedical discoveries. Members of the Global Alliance Translation Together¹⁷, are working in the field of translational science to understand the scientific and operational principles underlying each step of the translational process. Further development of that field is advanced by understanding the key characteristics of individuals who seek to uncover these principles to increase the efficiency and efficacy of translation. Translation Together, a newly launched international collaborative effort to advance translational innovation, have published²⁶ a consensus representation of the fundamental characteristics of a translational scientist. These encompass:
- Rigorous researcher
- Team player
- Boundary crosser
- Process innovator
- Domain expert
- Skilled communicator
- Systems thinker
Figure (3): Seven fundamental character traits of a translational scientist
Source: The Fundamental Characteristics of a Translational Scientist | ACS Pharmacology & Translational Science (original paper – Ref 25) or Translational Science Skills | National Center for Advancing Translational Sciences (nih.gov) (website with YouTube video). https://youtu.be/TnHLo-hCssg
Thus, the output of translational medicine (i.e. novel discoveries in biomedical research that are turned into solutions for the patient) depends on how well the process of translation is managed, while keeping the key characteristics for successful translation in mind from research start to marketing authorisation, and in particular during key decision points at pivotal stage gates (i.e. the decision to invest further in validation and regulatory compliant preclinical development in preparation of first-in-human clinical studies on the basis of key laboratory findings). Key characteristics for full translation of the research findings are that the output is:
- Feasible (technically achievable, realistic, doable);
- Useful (there is a need, a market, an end user);
- Scalable (provide a solution for the entire relevant population concerned, and that it can be reached);
- Exploitable (it can achieve impact, through return of societal value on the investment).
3. The need for translational medicine
Independent of its definition, what remains clear is the enormous need for translational medicine. A non-exhaustive list of arguments that expresses this need for translational medicine and, consequently, a (more) efficient process of translation enabled through translational science and high-quality research is depicted below.
From the patients’ perspective:
- The rapidly growing life expectancy²⁷ in most world populations has resulted in an increased prevalence of chronic disease for which treatments can be costly and prolonged.
- The continued rise in prevalence of certain diseases, such as Alzheimer’s disease, cancer or metabolic disease has resulted in a projected growth of healthcare spending²⁸.
- Environmental factors pose new health risks that need to be understood from a scientific mechanistic point of view and counteracted from a societal perspective (e.g. use of agrochemicals has been associated with enhanced risks of Parkinson’s disease)²⁹.
- Patients are better informed about their disease diagnosis, staging and prognosis, thus anticipating preventive measures, treatments and interventions that are tailored to their individual profile and needs to enhance their quality of life. (Anecdotal evidence).
- Patients are getting more organised in federations, charities and patient organisations, contributing to the democratisation of healthcare that demands cost-effective solutions through innovation. (Anecdotal evidence).
From the technological perspective:
- Key developments in genomics have led to novel opportunities in personalised medicine, an important theme in translational medicine. Improved procedures and exchange of knowledge has led to improved diagnoses and this has increased the requirements for treatments for newly identified, often rare, diseases. Technological developments (such as gene editing, artificial intelligence, big data analysis) have created new research fields that bring new opportunities and solutions for translational medicine³⁰.
- Providing an increased availability and access to data, e.g. electronic health records open up new possibilities for translational research, but need to be organised well to enable cross- and multi-disciplinary collaboration of high quality (i.e. that can be reproduced)³¹.
- The faster pace of technological advancements with innovative and alternative models to study medicine asks for dialogue between the regulatory agencies and the academic researcher³².
- The continuously changing landscape, with on the one hand large pharma becoming more risk averse and on the other hand a thriving biotech sector with (academic) spin-outs, causes a shift of ownership of the medicine development process, relying on an efficient translational medicine ecosystem to bring innovations to the patient³³.
- Emerging concepts such as the repurposing and repositioning of existing (generic) medicines and investigational drugs asks for new collaboration models, fit-to-task regulation and value-based pricing models.
4. Challenges and opportunities for translational medicine
Due to its complex landscape involving many actors, there are many challenges in the field of translational medicine that ultimately must enable public health models for disease prevention or treatment, with realistic resource settings to be successfully implemented. This is possible by identifying the means to create a supportive environment (ecosystem) for translational medicine and to develop novel methods for diagnosis, prognosis, and therapy for urgent and unmet global needs.
Knowledge of many fundamental aspects of biology in health and disease is still insufficient to automatically translate current findings reliably into new and more effective diagnosis, prevention tools and treatment. The goals of translational medicine can be attained only through continuous investment and advances in basic biomedical discovery coupled with efficient translational science in a continuum where dissemination of new knowledge in clinical and translational medicine can have beneficial effect on clinical practice.
One of the challenges of translational medicine is the continuous change of the landscape with changes in ownership of crucial parts of the medicine development process and with different actors entering the arena. Pharmaceutical industry, through mergers and acquisitions, create robust development pipelines and are essential powerhouses in drug development, but increasingly have focused on the later phases of the innovation chain, avoiding high(er) attrition rates in the riskier early part of the process. Historically, pharma industry had the full range of multidisciplinary research, expertise and resources in house to generate pipelines independent from competitors, but often leading to duplication of efforts. Nowadays, substantial innovation comes from biotech start-ups and academic spin-outs, where researchers are increasingly encouraged to become entrepreneurs and step into the arena to cover the early phases of drug development, the domain of translational medicine. These different actors in the innovation chain need to develop new/additional skills, rely on access to external resources, expertise and are dependent on new ‘truly collaborative’ organisational models.
From a technological, scientific and operational point of view, according to NCATS³⁴, researchers nationwide and across the globe face common barriers in translational research that can delay the development of new interventions for patients in need. Those barriers in translation have to be considered on a system-wide level to accelerate the development of treatments and preventive strategies. Predicting biological effects of drugs, chemicals and interventions carries uncertainty. Approximately 80 percent of investigational drugs fail in clinical trials because they are found to be unsafe or ineffective. More than 30 percent of promising compounds have failed in clinical trials because they are found to be harmful to human health (i.e., they have high toxicity), despite promising and costly preclinical studies in animal and cell models. Because these models often do not adequately represent human physiology, they frequently do not accurately reflect how patients will react to an investigational drug.
A major area of emphasis in translational medicine is the development of model systems for drug and toxicity testing that more closely resemble human physiology. Such advances could save enormous amounts of time and expense by preventing patients from being exposed to potentially harmful or ineffective investigational drugs in clinical trials. In addition, these models have the potential to provide useful information about the basic biology of disease and serve as improved testing platforms for predicting toxicity or other physiological processes as well as evaluating environmental chemicals.
The ‘downstream process’ towards the patient requires a rigorous approach with de-risking the therapeutic development using the right technologies and expertise. Involving the patient’s perspective early in the process helps to define the end product requirements to address the specific unmet medical needs as a guiding principle throughout the translational process
4.1 Quality and reproducibility
Reproducibility of research results is a fundamental component of science. The process of translational research involves conducting experiments to test and/or generate a hypothesis. The results of these experiments are collected, analysed and then shared with the wider research community through publication. Science progresses as hypotheses are further generated, tested, refined and validated, building on existing findings. The scientific progress requires that studies are rigorous and the findings reproducible. However, in a recent survey by the journal Nature, around 90% of respondents agreed that there is a “slight” or “significant” reproducibility crisis³⁵. Reproducibility has recently become a major talking point for multiple scientific domains: biomedical sciences, humanities and social sciences, computer sciences, psychology sciences, and is therefore a relevant issue in translational research as well. In December 2020, the European Commission published a scoping report³⁶ to increase the understanding of the lack of reproducibility in Europe and help design a suitable response in the context of EU Research & Innovation. Shortly thereafter, in February 2021, the ERIC Forum organised a two-day interdisciplinary workshop to evidence that reproducibility affects many research fields and requires multi-stakeholder efforts from funders, journals and researchers themselves to address the reproducibility crisis³⁸.
There is no single cause of and solution to irreproducibility. In some cases, poor experimental design, inappropriate analysis and questionable research practices can lead to irreproducible results. Cultural factors, such as a highly competitive research environment and the high value placed on novelty and publication in high-profile journals, may also play a part.
There is a consensus both in academia and industry, and in particular in the field of translational medicine, that there is a crisis involving the translatability of preclinical science to human applications and that most research findings are irreproducible or false³⁹,⁴⁰. However, scientific journals, societies, industry, regulators and academic research consortia increasingly embrace methods to make translational research more reproducible by adopting responsible science that improves the overall integrity, trustworthiness and quality of research.
4.2 The valley of death
In a detailed paper⁴¹, A. Seyhan outlines that the high attrition rates of drug development are only partly caused by reproducibility issues and that translatability of preclinical findings to human applications is dependent of many complex aspects. Poor hypotheses at the start of research programmes, irreproducible data analyses, ambiguous preclinical models, statistical errors, the influence of organizational structures, the lack of incentives in the academic setting, governmental funding mechanisms that look at different metrics than (long term) impact on patients, the clinical relevance of basic research, insufficient transparency, and lack of data sharing in research are all contributing to what is referred to as ‘the valley of death’.
Figure (4): The different phases of the translational process and the valley of death
Source: Ref. 31
The goals of basic research, or fundamental science, are aimed at advancing knowledge that is shared in scientific publications. On the other hand, goals of applied research, or development, focus on the generation of products with a specific utility value, where inventions are protected by patent applications as a mean to generate intellectual property, in a competitive setting. Historically, these two domains of basic and applied research have not been well aligned to facilitate the transition from the research to the development phase. The main reason for this disconnect is the lack of natural owners in this transition phase, which often involves costly method development, upscaling and validation of research results, with the aim to de-risk all elements towards full development of promising novel research findings. This is exacerbated by the often limited financial and societal incentives to promote the translation of research findings.
4.3 New developments (translational medicine today)
Despite the challenges due to its inherent complexity, translational medicine, including translational research and translational science, is becoming a recognised and more established discipline.
Figure (5): Number of scientific publications including the term “translational medicine”.
Source: https://esperr.github.io/pubmed-by-year/?q1="Translational Medicine"
The number of scientific publications in PubMed referring to “translational medicine” has increased almost 10-fold⁴² between 2010 and 2020, indicative of a wider recognition and interest by the scientific community. Governments and research councils also recognise the discipline of translation and need to differently organise ecosystems where different actors can collaborate in a more efficient way. The Dutch research council published an advisory report on how science can contribute to efficiency gains in medicines development through innovation. This report outlined the importance of
- achieving more patient centric research outcomes (e.g. through inclusion of patient reported outcomes or PROs in clinical studies);
- implementing personalised medicine;
- generating truly predictive models and;
- promoting equitable access to medicines in the market.
On the European scale, conventional disciplines are merging into multidisciplinary, “mission-oriented” endeavours⁴⁴ to avoid siloed research. The pharmaceutical industry landscape is continuously reshaping itself, and more open to pre-competitive approaches with novel collaboration models (e.g. initiated through the Innovative Medicines Initiative)¹⁵.
Technological developments bring new opportunities to translational medicine that are further leveraged by open data access, data sharing models and the creation of integrated databases, such as the human brain atlas⁴⁵ and the human protein atlas⁴⁶. Advanced research infrastructures are shared and getting more organised and rapid technological developments, such as gene editing with CRISPR-Cas9, reduced costs of genomic profiling, and promising methods based on artificial intelligence will continue to develop translational medicine. Finally, patient foundations, patient charities and patient organisations are getting better organised, with a stronger voice, thus playing a crucial role in the collection of high quality real world data that can flow back into translational research.
5. Achievements of translational medicine
The integrated approach described in this chapter has helped to translate the remarkable scientific innovations that occurred in the last years into health gains for the general population. This has been accomplished by:
- Using advances in physics and materials science which offer new approaches to study or diagnose medical conditions (e.g. organs on a chip, microfluidics assays).
- Helping in expediting the incorporation of novel endpoints into clinical testing, thereby shortening the duration of (early) clinical trials (e.g. adaptive trial designs, imaging read-outs supported by artificial intelligence methods for more sensitive, earlier detection).
- Facilitating the transfer of investigational medicines into the clinic, thereby leading to more rapid validation of new products and reducing costs associated with non-clinical testing (e.g. microdosing studies with minimal amounts of test substance supported by highly sensitive detection methods, such as mass spectrometry methods).
- Integration of multiple analytical platforms has enabled the field of (more) personalised medicine, using genomics, proteomics, metabolomic techniques, especially in the field of rare diseases, paediatric and geriatric medicine.
- New ways to conduct clinical trials have been sought. Innovative trial designs involving patients and allowing multiple companies to work together in integrated research platforms are under development⁴⁷.
Translational medicine, in enhancing the efficiency of biomedical discovery and application, rather than attempting to modify existing processes within the different disciplines, has come to serve as a unifying concept in the increasingly complex, specialised, and fragmented field of biomedical research. It has emerged based on the synthesis of information gained from multiple investigative sources. Owing to this approach, human biology and diseases are better understood and therapies, diagnostics and preventive measures are more rapidly identified and tested, which together result in improved patient treatment and outcomes.
The need remains to stimulate the development of a clearer vision for translational and clinical research, to ensure that these disciplines remain powerful engines of creativity.
Additional use cases to be considered for the factsheet:
- Covid-19
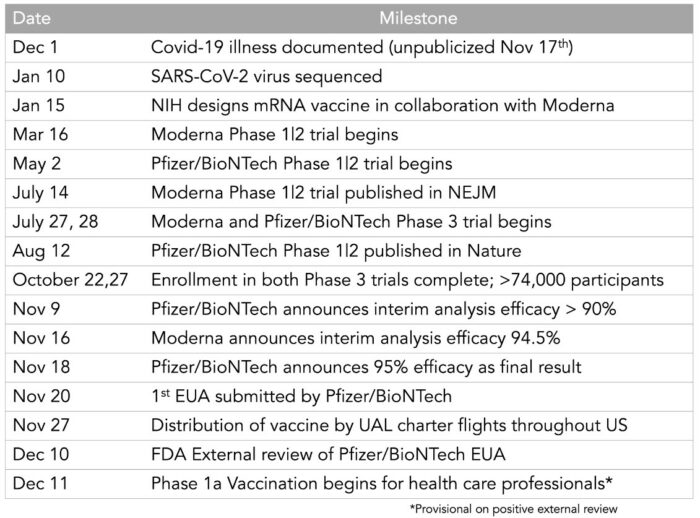
The Covid-19 pandemic has presented itself as a case where the translational process is compressed and maximally challenged by the parallel (’at risk’) planning of pre-clinical, clinical development and production activities. Although this is unrealistic in terms of financial risks, it will certainly provide new insights and ‘best practice’ guidance for the development of anti-viral therapies and preventative vaccines. Example: rapid development of novel mRNA vaccines and antiviral therapeutics. According to Eric Topol, Head of Scripps Translational Research Institute, this will go down in history as one of science and medical research's greatest achievements, perhaps the most impressive. He put together a timeline for 2020 of some key milestones to show how several years of work were compressed into months.
Table: Development timeline for the first Covid-19 mRNA vaccine
Source: https://twitter.com/erictopol/status/1332771238771630080?s=21
- CAR-T – Kymriah.
- Tisagenlecleucel - Wikipedia
- Oncology – personalised medicine:
- Pembrolizumab - Wikipedia
- Drug repurposing of axitinib
- Repurposing drugs in the spotlight | Centre for Cancer Biomarkers CCBIO | UiB
- Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation - PubMed (nih.gov)
6. Attachments
- Fact Sheet: Examples of translational medicine research
Size: 110,067 bytes, Format: .docx
Examples of translational medicine.
- Presentation: Translational Medicine: An Introduction
Size: 633,061 bytes, Format: .pptx
Introduction to translational medicine.
- The Fundamental Characteristics of a Translational Scientist:
- https://pubs-acs-org.vu-nl.idm.oclc.org/doi/10.1021/acsptsci.9b00022
[glossary_exclude]Footnotes
1 translational medicine | Britannica
2 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5408839/pdf/S2059866116000108a.pdf
3 National Institute of Health (USA)
4 https://ncats.nih.gov/translation/spectrum
5 Clinical Research and Translational Medicine - Central Clinical School (monash.edu)
6 Autoantibodies: Early diagnosis & patient stratification (selectscience.net)
7 Elias Zerhouni. Nat Biotechnol 29, 188 (2011)
8 a) http://stsiweb.org/index.php/translational_research/; b) http://stsiweb.org/index.php/about/leadership/founders/; c) https://www.mobihealthnews.com/content/dr-eric-topol-talks-ais-potential-personalize-medicine-curb-mistakes-bring-back-human-touch
9 https://doi-org/10.1111/j.1752-8062.2009.00160.x
10 https://www.sciencedirect.com/science/article/pii/S1359644616000192
11 https://www.novartis.com/our-science/translational-medicine
12 https://www.ascpt.org/Resources/Knowledge-Center/What-is-Translational-Medicine
13 Mission and scope | Science | AAAS (oclc.org)
14 https://en.wikipedia.org/wiki/National_Center_for_Advancing_Translational_Sciences
17 Homepage | IMI Innovative Medicines Initiative (europa.eu)
18 Innovative Health Initiative | IMI Innovative Medicines Initiative (europa.eu)
19 Translation Together – Global alliance for Translational Medicine
20 National Center for Advancing Translational Sciences | (nih.gov)
23 Research | Therapeutic Innovation Australia Ltd
24 Fundação Oswaldo Cruz (Fiocruz): Ciência e tecnologia em saúde para a população brasileira
25 Japan Agency for Medical Research and Development (amed.go.jp)
26 https://doi.org/10.1021/acsptsci.9b00022
27 Life Expectancy - Our World in Data
28 https://ourworldindata.org/health-meta
29 Dorsey, E.R. and Bloem, B.R. JAMA Neurol. 2018;75(1):9-10. doi:10.1001/jamaneurol.2017.3299
30 De Maria Marchiano et al. Translational Research in the era of precision medicine: where are we and where will we go J Pers Med 2021, 11(3):216
32 Welcome to STARS! - Stars (csa-stars.eu)
33 Romasanta et al. Innovation in pharmaceutical R&D: mapping the research landscape Scientometrics 2020, 125, 1801-1832
34 https://ncats.nih.gov/translation/issues
35 crisis http://www.nature.com/news/1-500-scientistslift-the-lid-on-reproducibility-1.19970
36 https://op.europa.eu/en/publication-detail/-/publication/6bc538ad-344f-11eb-b27b-01aa75ed71a1
37 The ERIC Forum Implementation Project is a Horizon2020 project which brings together the ERIC community to strengthen its coordination and enhance its collaborations. ERIC: The European Research Infrastructure Consortium (ERIC) is a specific legal form that facilitates the establishment and operation of Research Infrastructures with European interest.
39 Ioannidis JP. Why Most clinical research is not useful. PLoS Med. 2016;13:e1002049.
40 Ioannidis JPA. Why most published research findings are false. PLoS Med. 2005;2:696–701.
41 https://transmedcomms.biomedcentral.com/articles/10.1186/s41231-019-0050-7
42 https://esperr.github.io/pubmed-by-year/?q1="Translational Medicine"
44 Missions in Horizon Europe | European Commission (europa.eu)
45 https://portal.brain-map.org/
46 https://www.proteinatlas.org/
47 EU-PEARL - Innovative Patient Centric Clinical Trial Platforms[/glossary_exclude]