Safety communication
What is safety communication?
Safety communication is put in place to communicate adverse reactions or any safety findings to regulators, healthcare providers and patients. It is a broad term that includes different kinds of information, including the information contained in the product information (such as Summary of Product Characteristics (SmPC), Package Leaflet (PL), and package labelling) and in public assessment reports. Safety communications are provided by marketing authorisation holders (MAHs) and health authorities (HAs), and may include restrictions, contraindications (situations where a medicine must not be used because it may be harmful to the patient), dose limitations, warnings, and recommendations.
Safety communication aims to:
- Prevent patients from experiencing adverse reactions;
- Provide timely, evidence-based information on the safe and effective use of medicines and appropriate clinical management of patient treatment;
- Facilitate changes to healthcare practices (including self-medication practices) where necessary;
- Change attitudes, decisions, and behaviours in relation to the use of medicines;
- Support risk-minimisation behaviour; and
- Facilitate informed decisions on the rational use of medicines.
Safety communication is a public health responsibility, essential for achieving the objectives of pharmacovigilance and promoting the rational, safe, and effective use of medicines, preventing harm from adverse reactions (ADRs), and contributing to the protection of patients and public health.
Why is safety communication important?
Safety communication is a key tool in preventing adverse reactions (ADRs).
The communication of safety information can provide:
- Knowledge from previous experience that has been reported and analysed.
- Recommendations about the appropriate clinical management of patient treatment.
Safety communication directed towards patients can also:
- Influence patient attitudes to certain medicines.
- Help to prevent possible side effects.
- Reduce medication errors.
- Inform patients about the safe use of their medicines.
How is safety information gathered after a medicine is on the market?
After a medicine is given a marketing authorisation (MA) and starts being administered to patients, it is critical that the safety profile of the medicine continues to be monitored. Although the benefit-risk balance of the medicine is carefully assessed before marketing authorisation is granted, sharing and monitoring data over long periods of time and in larger populations gives a better understanding of the medicine’s benefit-risk balance in a real-world context and helps to detect very rare side effects. It is therefore vital that the safety of all medicines is monitored throughout their use.
[glossary_exclude]Marketing authorisation holders (MAHs) are responsible for collecting, reviewing and analysing spontaneous case reports of suspected adverse reactions to medicines by patients and healthcare professionals, in scientific literature and elsewhere during post-marketing surveillance of medicines. They must share adverse event reports on an expedited basis, if urgent safety issues are identified, such as serious adverse events. In the EU, MAHs must report this information using the electronic EudraVigilance system. EudraVigilance is a centralised European database of suspected adverse reactions to medicines that are authorised or being studied in clinical trials in the European Economic Area (EEA). The European Medicines Agency (EMA) operates the system on behalf of the European Union (EU) medicines regulatory network. The EMA Pharmacovigilance Risk Assessment Committee (PRAC) evaluates signals from EudraVigilance and may recommend regulatory action as a result.[/glossary_exclude]
Yellow Card Scheme – an example of gathering safety information
In the United Kingdom, the Medicines and Healthcare Products Regulatory Agency (MHRA) and Commission on Human Medicines (CHM) run a spontaneous ADR reporting scheme called the ‘Yellow Card Scheme’. It acts as an early warning system for the identification of previously unrecognised adverse reactions. Patients can use the Yellow Card Scheme to report any side effects they experience while using a medicine, and carers or parents can use it to report any side effects on behalf of a patient in their care. Healthcare professionals can also use the scheme to report possible adverse effects of a medicine.
The information gathered through the Yellow Card Scheme can help the MHRA to identify and refine the understanding of risk factors that may affect the clinical management of patients. Information on the nature of the benefits and risks of the medicine can help healthcare professionals and patients make informed decisions about treatment options and the management of ADRs should they occur.
[glossary_exclude]Web-RADR – an app to gather safety information
The IMI WEB-RADR project (https://web-radr.eu) has worked with national health authorities to develop three smartphone apps enabling patients, caregivers, and healthcare professionals to report adverse drug reactions (ADRs) and receive up-to-date information and news alerts.
- UK (Yellow Card) – launched 14 July 2015.
- Netherlands (LAREB) – launched 29 January 2016.
- Croatia (HALMED) – launched 18 May 2016.
Key features:
- A convenient alternative to paper or electronic ADR reporting forms.
- Easy to use for reporting side effects.
- Free to use for everyone on iOS and Android.
- Enables users to:
-
- create a ‘watch list’ of medications to receive official news and alerts,
- view numbers of reports received by the National Competent Authority (MHRA, LAREB or HALMED) for medicines of interest,
- see immediately that the ADR report has been accepted,
- submit updates to ADR reports already submitted,
- view previous ADR reports submitted through the app.[/glossary_exclude]
[glossary_exclude]How is new safety information communicated?
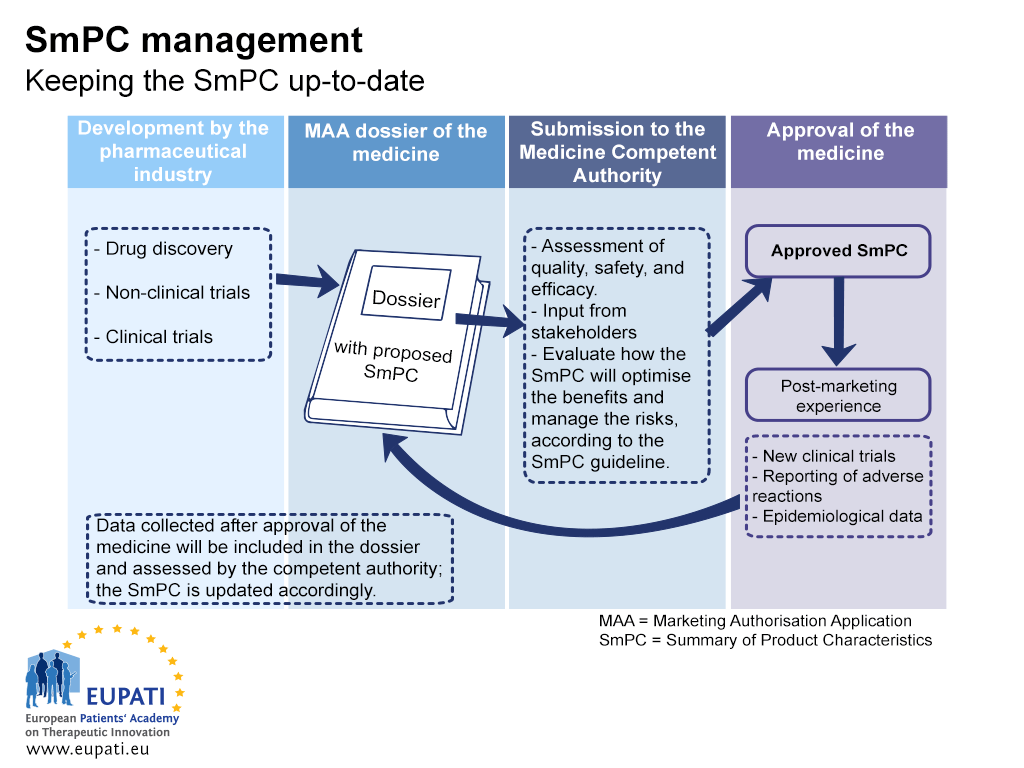
On its own, safety reporting does not improve safety – it is the response to these reports that leads to a change that will improve safety. For instance, when a new safety concern is identified for a medicine, the safety information that is provided in the Package Leaflet (PL) and the Summary of Product Characteristics (SmPC) is updated.
- The Summary of Product Characteristics (SmPC) must be kept up-to-date throughout the lifecycle of a medicine.
Depending on the severity of new safety information, different channels of communication can be considered. These include but are not limited to:
- Direct communication to healthcare professionals (DHPC) by the MAH or the national competent authorities.
- Documents published in simple language (for instance in a question and answer format) to help patients and the general public understand the scientific evidence and regulatory activities relating to the safety concern.
- Press communication – including press releases and briefings intended primarily for journalists. Journalists are an important means of reaching a wider audience. However, in cases where there is also direct communication to healthcare professionals, the healthcare professionals should receive the information before or at the same time as the communication to the press.
- Websites of national competent authorities and MAHs, which can provide easily accessible and understandable information. New legislation on pharmacovigilance states that the EU will play a more important role in the sharing of safety information online, with the creation of an EU medicines web portal containing information on all medicines authorised in the EU and links to national medicines web portals.
- Other web-based communications such as social media.
- Bulletins and newsletters provided by competent authorities.
- Inter-authority communications, such as ‘line-to-take’ documents prepared by the authorities to assist their own staff in responding to external enquiries or in communicating on specific safety issues.
- Systems put in place by the MAHs or the competent authorities to respond to enquiries from individual members of the public.
- Scientific journals and professional bodies’ publications.
- Patient organisations’ websites or publications.
Communicating with healthcare professionals and patients is an important part of pharmacovigilance. These processes can be global or local. For example, some clinics and hospitals have special schools for patients that have specific objectives, such as getting patients’ opinions and feedback on an established method of treatment for a disease.[/glossary_exclude]
[glossary_exclude]What is the role of patients in safety communication?
It is important to incorporate patient experience and knowledge at different stages throughout the process of developing, assessing, licensing, and monitoring medicines. Patients should be involved in identifying and reporting treatment complications and adverse events.
Patient are now involved in these activities in the EMA. With regards to safety communications, for example:
- A patient representative now sits as a full member in the Pharmacovigilance Risk Assessment Committee (PRAC);
- A pilot project has been launched by the EMA on involving patients in discussions in the Committee for Medicinal Products for Human Use (CHMP); and
- Patients are now consulted on issues such as disease management, quality of life, and the feasibility of risk management programmes.
Reporting adverse events is a key responsibility. The collection, review, and analysis of information on adverse events can:
- Result in a change of product information and the benefit-risk profile of a medicine.
- Help identify risks.
- Provide information about where the healthcare system may be unsafe.
Patients can be active in the reporting of adverse events – for instance through schemes such as the Yellow Card System or using a WEB-RADR app. Patient focus in reports of suspected ADRs differs from that of healthcare professionals – although there is a reasonable amount of overlap – and can generate new potential safety signals and describe suspected ADRs in enough detail to provide useful information on likely causality and the impact on patients’ lives (1).[/glossary_exclude]
[glossary_exclude]References
- Avery AJ, Anderson C, Bond CM, Fortnum H, Gifford A, Hannaford PC, et al. Evaluation of patient reporting of adverse drug reactions to the UK Yellow Card Scheme: literature review, descriptive and qualitative analyses, and questionnaire surveys. Health Technol Assess 2011; 15 (20). http://www.ncbi.nlm.nih.gov/pubmed/21545758[/glossary_exclude]
A2-5.28-V1.0