Risk communication in medicines
Introduction
Almost all medicines will have some level of risk attached to them. In the majority of cases, this risk may be negligible, but any identified risks (such as potential adverse effects, interactions) are listed in the package leaflet. Health Authorities and Marketing Authorisation Holders (MAHs) are obligated to monitor and improve all medicines in use. ‘Risk communication’ is a part of this obligation.
What is risk communication?
Risk communication can be defined as an open two-way exchange of information and opinions about harms and benefits, with the aim of improving the understanding of risk and of improving decisions about the use of medicines. Risk communication should therefore cover:
- The probability of the risk occurring
- The importance of the adverse event being described
- The effect of the event on the patient.
During the development of a medicine, risk management must be addressed in a Risk Management Plan (RMP), which includes both the minimisation of and communication about potential risks.
A medicine is authorised on the basis that, at the time of the authorisation, the benefit-risk balance has been judged to be favourable in the target population for the specified indication(s). However, not all actual or potential risks will have been identified at the time when an initial authorisation is given. In addition, efficacy in the clinical trial setting may not reflect the true effectiveness of the medicine in every-day medical practice. The benefit-risk balance of a medicine as assessed at the time of authorisation will inevitably change after the medicine is authorised for use.
It is therefore a shared responsibility between the health authorities and the Marketing Authorisation Holder (MAH) to have a system in place that allows the:
- Identification and analysis of risks
- Performance of the benefit assessment
- Re-evaluation and characterisation of the benefit-risk assessment
- Implementation of activities that minimise the risk in order to protect public health
- Communication of the risk
Minimising the risk
Patients and healthcare professionals have access to several sources of controlled and standardised formats of information about the medicines and their risks. These are the Package Leaflets (PL) and the Summary of Product Characteristics (SmPC).
In addition to the PL and the SmPC, other elements can help to manage the risk of using medicines in an uncontrolled way, for instance:
- The package size
A small pack size can be useful in controlling risk, especially if overdose is thought to be a major risk. - The legal status of the medicine
Controlling the conditions under which a medicine may be made available can reduce the risks associated with its use or misuse. This can be achieved by controlling the conditions under which a medicine may be prescribed, or the conditions under which a patient may receive a medicine without a prescription (also called over-the-counter (OTC)).
The process and timing of risk communication
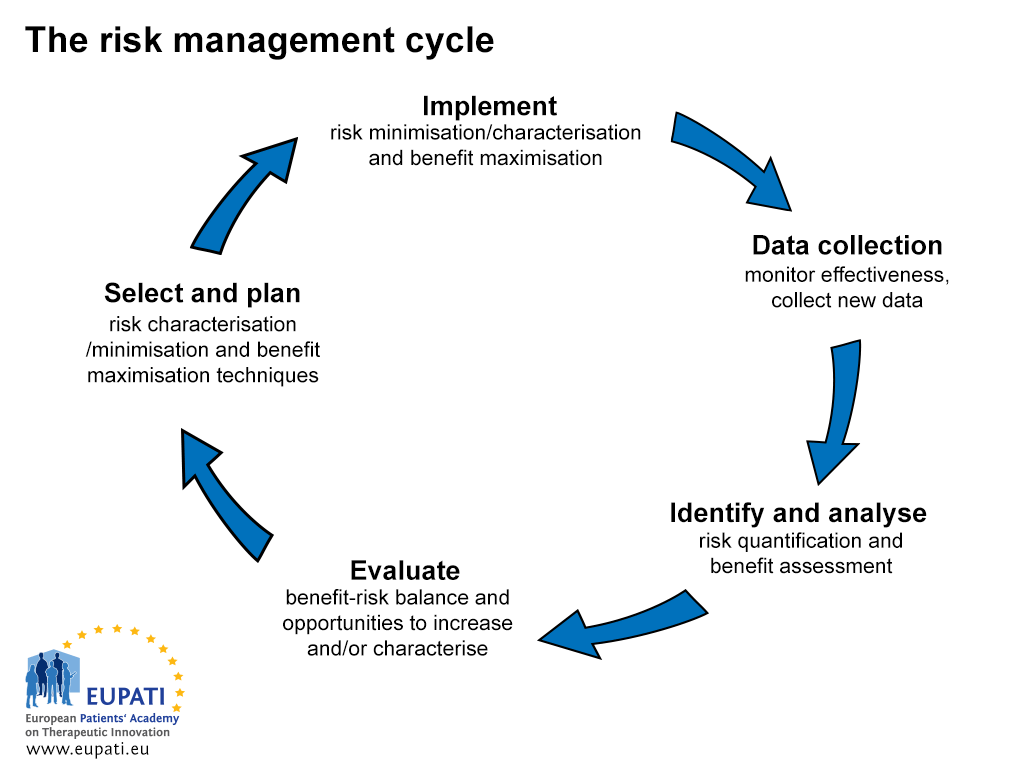
The process of risk management and communication is a five-step process (see Figure 1).
- There are five steps in the risk management cycle.
The risk management and communication process starts during the data collection phase with an initial alert of a ‘suspicious situation’. The MAHs of a medicine are responsible for ensuring the constant monitoring of the risks of their medicines in compliance with relevant legislation. They are then required to report the results to the appropriate competent authorities.
The MAH is also responsible for taking all appropriate actions to minimise the risks of the medicine and maximise the benefits. This includes ensuring the accuracy of all information produced by the company in relation to its medicines and actively updating and promptly communicating new information as it becomes available.
The second stage of the risk communication process is to identify and analyse the risk and assess it in relation to the benefits. This takes some time, and means that there is a period of uncertainty that requires ‘precautionary decisions’. The next phase is to evaluate and quantify the risks and benefits. This is followed by the selection of a plan for minimising risks and maximising benefits. The final stage is the implementation of the plan.
Throughout the communication process, it is important that clear and consistent messages are provided in a timely manner. The principles of safety/risk communication should be applied. These principles include:
- Adequate coordination and cooperation between the different parties involved
- Messages should be relevant, clear, accurate and consistent, reaching the right audiences at the right time for the to take action
- Communication should be tailored to the appropriate audience by the use of appropriate language
- Risk communication should be presented in the context of the benefits of the medicines and should include available and relevant information about adverse reactions
- Any uncertainties related to the safety concern should also be addressed
- Information on competing risks should be included when appropriate
- The most appropriate quantitative measures should be used when describing and comparing risks
- Follow-up communication with complementary information should be issued on the resolution of the safety concern
- The effectiveness of the communication should be evaluated
There are challenges in risk communication. Barriers such as poor numeracy, imprecise language, and the framing (how the risk is presented) can have an impact on the perception of the information.
Health literacy is one of the biggest challenges of risk communication. Both patients and professionals can have difficulty interpreting, incorporating, and/or remembering risk information, especially the statistical aspects.
For patients, the majority of medicines will be prescribed by doctors and dispensed by pharmacists. Management of benefits and risks by patients will primarily involve:
- Complying with treatment schedules and recommendations
- Being aware of important risks and what actions to take
- Reporting any untoward effects to their doctor, pharmacist, and national competent authority
However, depending on the country, there are some medicines that may be bought directly without guidance from healthcare professionals (over-the-counter (OTC) medicines). Patients need to understand the potential benefits and risks of these products, and what measures they need to take to use the medicines safely and effectively.
Patient involvement in risk communication
The role of patients in risk communication is very important. They can participate in different ways:
- By collecting safety information about their experience with medicines – this is important, as they are directly effected
- By being consulted when drafting the safety or risk messages and for pre-testing such messages o ensure they are relevant, clear, and understandable
- By being involved in the development of a number of documents made publicly available – for instance, the European Public Assessment Report (EPAR) summaries, package leaflets, and safety communications
- By having regular dialogue with the different stakeholders
- Through input and dissemination of risk communications in media (social media, press releases, etc.)
Further Resources
- Edwards, A. (2004) ‘Flexible rather than standardised approaches to communicating risks in health care’. British Medical Journal Quality & Safety, 13(3): 169-170. Retrieved 11 September, 2015, from http://qualitysafety.bmj.com/content/13/3/169.full.pdf+html
- Pfizer Inc. (2011). Understanding risk. Retrieved 12 July, 2021, from https://web.archive.org/web/20140202170937/http://www.pfizer.com/files/health/medicine_safety/1-2_Understanding_Risk.pdf
Attachments
- Risk-Communication-in-Medicines
Size: 495,341 bytes, Format: .pptx
A presentation describing risk communication process in medicines, which can be adapted for own use.
A2-5.27-v1.1