Last update: 11 July 2023
Introduction
A collaboration of Genzyme, International Pompe Association (IPA); UK, Dutch, and US Pompe patient organisations, Erasmus Medical Center and Duke University to establish appropriate measures to ensure the data in clinical trials is robust and will satisfy regulatory and payer requirements.
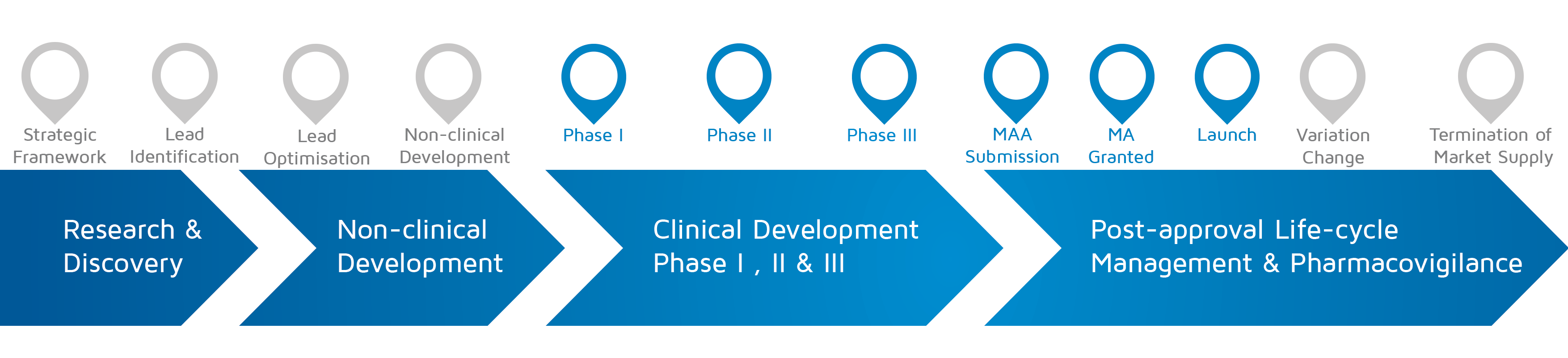
- When does it happen? – Phase I-II-III, MAA submission, MA granted, Launch
Description of the case
Pompe disease is a rare inherited neuromuscular disease due to deficiency of a lysosomal enzyme. Babies with <1% of GAA enzyme present as the infantile onset (IO) form and usually die within the first year of life, while individuals with some residual GAA activity may present from infancy to late adulthood with neuromuscular weakness, ambulatory and respiratory issues. Work carried out at Erasmus Medical Centre (EMC) and Duke University in the 1990’s with knock-out Pompe mice showed promise with enzyme replacement therapy as a treatment. Clinical and manufacturing development was discussed on a regular basis jointly with the academic centres and patient organisations (PO). Clinical trials first began with the IO babies due to the extreme rapidity of disease progression. However due to the rarity of the disease (~1:40.000 births) recruitment was challenging. The POs assisted in disseminating information about the trials, locating patients around the globe, finding lodging, parent support, etc. For the trials in children and adults, POs assisted in recruitment, review of assessments with Genzyme and investigators, and encouraging retention in these long placebo controlled trials (18 months) even after approval. A patient representative of IPA also presented at the oral explanation at the EMA (a first).The IPA in collaboration with EMC (supported financially by Genzyme) developed a patient reported outcome survey independently from industry years before treatment was available which has proven to be important in supporting reimbursement discussions.
Type(s) of patient (advocates) involved
- Patients with personal disease experience.
- Expert patient / patient advocate with good expertise on disease and good R&D experience.
- Professional patient advocate.
Benefits of patient involvement
There is regular communication with POs for rare diseases on our development plans as well as some reviews of protocol assessments. This has become much more difficult recently with the rules of conduct that have been implemented. We do not (yet) have the patient collaboration implemented in standard operating procedures.
Challenges and barriers
Development teams on the programs are given very strict timelines to complete protocols and get trials moving. There is often resistance in these teams to add another layer (on top of senior management from science, development, regulatory, safety, toxicology, clinical pharmacology, etc.) of input into protocol development. By ensuring timely input from the PO and illustrating the benefits in the long-run in terms of recruitment, fewer screen failures, better completion of assessments etc., one can convince internal project teams that this is worthwhile. Another challenge in some countries is the difficulty of direct contact; in that case we asked the investigator to contact the national PO and review the protocol with them for input. Internal concerns about maintaining confidentiality was overcome with a confidentiality agreement with PO, which allowed for free and frank communication. Lastly, the IPA patient survey, although it has provided valuable Patient Reported Outcomes (PRO) and many publications, it is not considered as credible to regulatory authorities and payers due to lack of source data verification, a lesson which could be applied in the future.
Learnings
Lessons learned:
- Establish a trio of academic experts, PO and internal project doctor with rules of communication at the start.
- Ensure that a PRO instrument is created and validated for the disease (what is the most important thing for the patient), with appropriate measures to ensure the data is robust and will satisfy regulatory and payer requirements.
- Start a natural history study, with the PRO, years before the treatment will be available in order to be able to compare.
- The most valuable input into the protocol is reviewing assessments, their feasibility etc. and should be standard.
- Keep community appropriately informed by providing program updates for dissemination through PO.
A3-pomp-V1.0
Attachments
- case-report-Orphan-disease-v1-EN
Size: 482,562 bytes, Format: .pdf
An infographic describing a collaboration between two patient organisations (International Pompe Association (IPA); UK, Dutch, and US Pompe patient associations), Genzyme and investigators from the Erasmus Medical Centre (EMC) regarding an orphan disease.