Last update: 11 July 2023
Today you will see our patient involvement report on successful patient involvement, make sure you have a read.
- A screenshot of a sample Patients Involved Case Report
In the coming weeks we shall share patient involvement reports on trials and projects with successful patient involvement. Be sure to check back each week to see the new story. You will see that these case studies are from a wide range of diseases areas, and that patient advocates and patient experts were involved in the lots of different stages.
The definition of success is also not always entirely clear-cut. In some cases, for example in the field of HIV/AIDS where I come from, success was sometimes stopping a trial, or just making sure that one or the other arm of a clinical study was modified to meet the patients’ real-life needs more.
In other cases the end result was the creation of a patient advisory group that could effectively intervene and provide input into clinical trials.
One thing is common in all of them: They bear witness to the countless possibilities and opportunities that are there for patients to be involved, even if it takes a lot of time and effort! Some groups or disease areas might be more ahead, others are just stretching their wings in the world of patient activism. This collection of good practices, which does not stop short of admitting mistakes or failures as well, can be seen as both a motivational tool and some handy help for patient advocates.
Do you have a story you’d like to share? Contact the content team with a description of your case of patient involvement, making sure to include all required information so that it can be published. Help us grow our library of patients involvement reports!
Information required for patient involvement reports:
Partners Involved
Type of patient (advocates) involved (Patients with personal disease experience, and/or Expert patient / patient advocate with good expertise on disease, but little R&D experience, and/or Expert patient / patient advocate with good expertise on disease and good R&D experience, and/or, Other, describe here: )
Description of the case (how were patients involved in the R&D project? What was the objective? (200 words max)
Benefits (how has this collaboration improved R&D process(es) and the R&D outcome(s) or triggered R&D organisational change) (150 words max)
Challenges and barriers (and how you have overcome them, or which ones were unresolved) (150 words max)
Discussion and learnings for you and EUPATI (what would you do differently next time, what are external factors that should change) (150 words max)
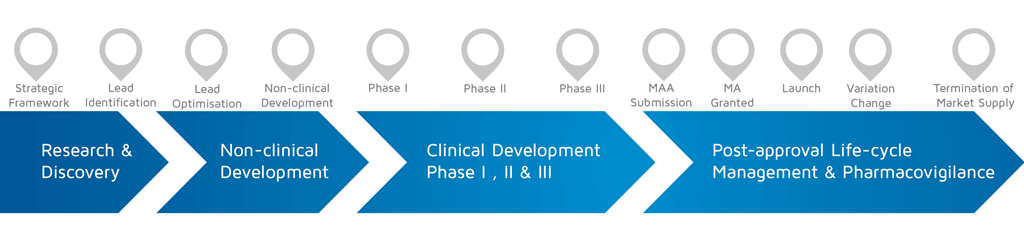
What phase of development did the involvement take place? (choose from the diagram below)
- When does it happen?
Send your story to [email protected]
Tamás Bereczky
European AIDS Treatment Group