Mini-course Starter Kit – Community Advisory Boards
Introduction
This EUPATI starter kit is designed for patient involvement in community advisory boards.
EUPATI Mini-course starter kits have been derived from content found in the EUPATI toolbox and EUPATI Patient Expert Training Course. The starter kits are thought to address roles that patients play in medicines development for example those shown in the figure below.
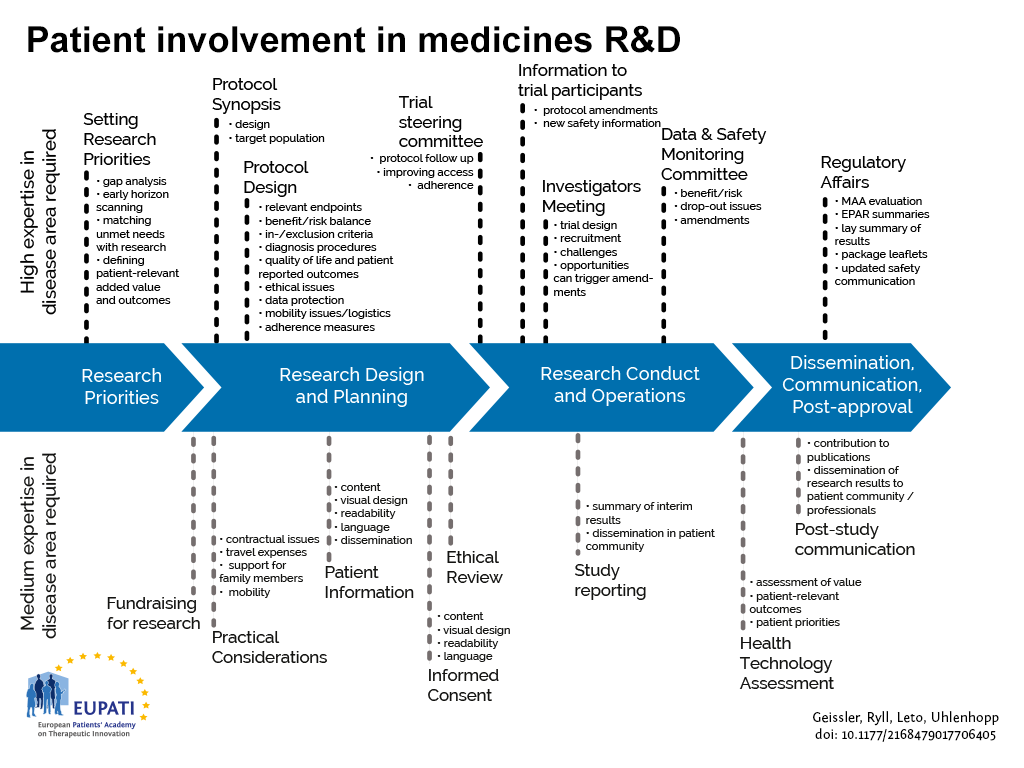
The starter kits provide you with links to relevant background reading in the toolbox and associated PowerPoint slide decks and media in order to prepare a single or multi-day training on the subject. Each of the starter kits contains a selection of PPT slides which you may use to educate patients/advocates about the “basics” in that area, e.g. in a two-hour to one-day seminar.
The starter kits are based on existing content from the EUPATI Toolbox, plus additional links to add-on Toolbox material. None of the “starter kits” are “ready-made course” modules – they are a ready-to-reuse resource for an experienced trainer to prepare and execute a course. You will need to edit them and put them into context.
Before you begin please download and review the ‘Manual for Trainers’: A manual for trainers describing how to use the EUPATI mini-course starter kits to create trainings on patient involvement.
Community Advisory Boards
This starter kit provides background reading, slides, videos, and quizzes to create training for patients who intend to become involved in Community Advisory Boards. In medicines development, a Community Advisory Board (CAB) is a group of patient representatives that serves as a link between a community and researchers. Within clinical development a CAB may review clinical trial protocols and monitor clinical trials and help teach the community about them. The CAB model has also been implemented in areas such as policy making and Health Technology Assessment (HTA).[glossary_exclude]
Core reading
- The Impatient Patient – A discussion of patient involvement in novel forms of knowledge production – a case study of the European Community Advisory Board on HIV/AIDS
- What are Community Advisory Boards?
Size: 434,918 bytes, Format: .pptx
A presentation describing what a Community Advisory Board is which can be adapted for own use. - How to Organise a Community Advisory Board
Size: 374,094 bytes, Format: .pptx
A presentation describing how to organise a Community Advisory Board which can be adapted for own use. - A Case-Study of the HIV European Community Advisory Board
Size: 607,848 bytes, Format: .pptx
A presentation describing a case study of the HIV European Community Advisory Board which can be adapted for own use.[/glossary_exclude]
Terms of use – Creative Commons
Remember that all educational content provided by EUPATI is released under a Creative Commons License, which also applies to all derivatives of it!
You can read more about the use of EUPATI content on the Creative Commons page.
Use of the EUPATI logo
The EUPATI logo is protected by trademark and owned by the European Patients Forum.
Except for the limited purpose of indicating that work is created or licensed by EUPATI (European Patients Academy for Therapeutic Innovation), or collaboration with EUPATI, the European Patients Forum (EPF) does not authorise the use, by any party, of the trademark “EUPATI” or any related trademark or logo of EUPATI without the prior written consent of EPF. Any permitted use will be in compliance with EUPATI’s then-current trademark usage guidelines, as may be published on its website or otherwise made available upon request from time to time.
A2-SK-community-advisory-boards-V1.0