Key principles of pharmacology
Introduction
Pharmacology is the study of how a medicine works, how the body responds to it, and the changes that occur over time. Non-clinical pharmacological studies allow scientists to compare a medicine’s beneficial effects with its negative (toxic) effects. This comparison is important so that a thorough benefit-risk analysis can be made before proceeding to test the medicine in clinical (human) studies. If the medicine does proceed to the clinical phase, data gathered during non-clinical pharmacology and toxicology studies help to determine the dosage of medicine given to volunteers in the first clinical studies (first-in-human).
Pharmacology is divided into two key areas: pharmacokinetics and pharmacodynamics. These are explained in more detail below.
Pharmacokinetics
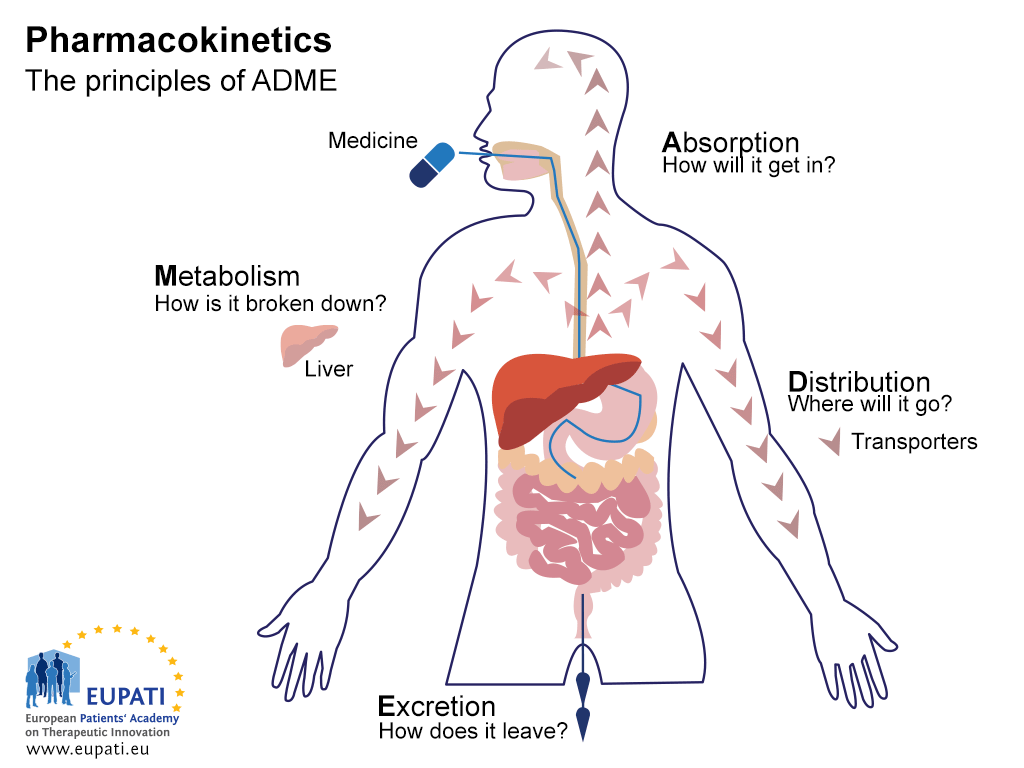
Pharmacokinetics (PK) is the study of the effect the body has on a medicine.
The acronym you will find in every textbook associated with pharmacokinetics is ADME:
Absorption: How the medicine gets in to the body
Distribution: Where the medicine goes in the body
Metabolism: How the body chemically modifies the medicine
Excretion: How the body eliminates the medicine
- The key principles of Pharmacokinetics – the study of the effect the body has on a medicine – are represented in the acronym ADME.
Data gathered during pharmacokinetic studies provide information about what happens to a medicine in the body over time. Scientific and mathematical models based on this information help to understand and predict the journey of the medicine and its metabolites through the body. This allows scientists to assess the relationship between the medicine’s beneficial and toxic effects, and to predict the safety/tolerability of the medicine in humans. Data gathered during pharmacokinetic studies are thus essential for determining dosing schedules in clinical trials.
Pharmacodynamics
Pharmacodynamics (PD) is the study of the effect of a medicine on the body.
There are two ways a medicine can affect the body:
- A medicine can change conditions within the body, or
- A medicine can interact with specific parts of the body on a cellular or sub-cellular level.
The primary objective of pharmacodynamic studies is to gather information on how the medicine affects the body (for instance, what receptors it activates). This allows scientists to assess the efficacy of the medicine – that is, whether or not the medicine is having the desired effect on the target, and if so, how strong that effect is. It also allows a better understanding of the relationship between the concentration of the medicine in the body and the strength of its effect.
Pharmacodynamic studies are crucial for the safety assessment of a medicine. They identify any undesirable effects that the medicine has and investigate the range of doses at which the desired effect of the medicine on the body occurs (therapeutic dose range).
Attachments
- Presentation: The Key Principles of Pharmacology
Size: 972,680 bytes, Format: .pptx
This presentation explores the key principles of pharmacology: pharmacokinetics and pharmacodynamics.
A2-3.06-v1.4