HTA systems in Europe
Introduction
Health Technology Assessment (HTA) is a multidisciplinary process that summarises information about the medical, social, economic, and ethical issues related to the use of a health technology in a systematic, transparent, unbiased, and robust manner. Its aim is to inform the formulation of safe and effective health policies that are patient focused and seek to achieve best value. Despite its policy goals, HTA must always be firmly rooted in research and the scientific method.
When considering HTA within the area of medicinal products, it is helpful to know how medicines are authorised and to have a basic appreciation of the life-cycle of a product and the processes that lead to a Marketing Authorisation (MA). It is also helpful to know how HTA fits into reimbursement or insurance coverage schemes, depending on the country. The pharmaceutical company must conduct high-quality, randomised clinical trials and submit an application dossier to the relevant regulatory authority. Once a product has been awarded an MA based on safety, quality, and efficacy, the product can enter the market (may be sold). To ensure widespread access to necessary treatments for patients, it is often necessary for the product to be covered by a national healthcare system or insurer. This would mean that the product may be included in the appropriate national reimbursed medicines list or insurance coverage.
At the same time, these institutional payers have to manage access to innovative treatments within a finite budget. Due to these constraints, payers want to ensure that they are paying for new technologies that offer real improvements to patient outcomes. This is where HTA comes in, as its fundamental role is to determine the added therapeutic value (in terms of health outcomes for patients) of the new technology in comparison to the current standards of care.
A useful starting point is to understand which organisations are the main actors in this process. In Europe, there are various bodies responsible for both pharmaceutical and non-pharmaceutical Health Technology Assessment (HTA). The structure, function, remit, and approaches of these bodies vary according to the different health systems and political structures they operate in.
Some examples of HTA bodies for pharmaceutical assessment in Europe include:[glossary_exclude]
- France – Haute Autorité de Santé (HAS) – http://www.has-sante.fr
- Germany – Gemeinsamer Bundesausschuss (GBA) – https://www.g-ba.de/
- Scotland – Scottish Medicines Consortium (SMC) – scottishmedicines.org.uk/Home
- Sweden – Tandvårds- och läkemedelsförmånsverket (TLV) – https://www.tlv.se/in-english.html[/glossary_exclude]
Note that in Germany, the evaluation component of HTA is carried out by IQWIG (Institute for Quality and Efficiency in Health Care), while the appraisal component and decision-making is carried out by the GBA. Note also that in some European countries, the HTA body also conducts evaluations of non-pharmacological interventions such as devices, surgical procedures, and (in some cases) public health interventions. These include:[glossary_exclude]
- Norway – FHI (Norwegian Institute of Public Health (NIPH), Folkehelseinstituttet) – https://www.fhi.no/en/
- Sweden – SBU ( Swedish Agency for Health Technology Assessment and Assessment of Social Services) – http://www.sbu.se/en/[/glossary_exclude]
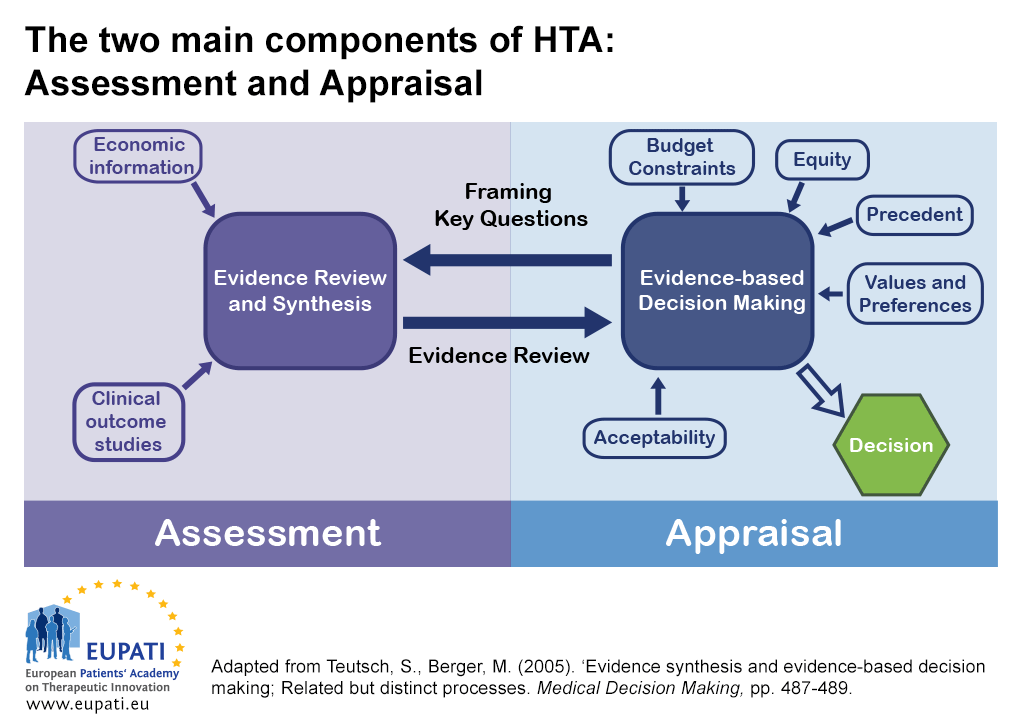
There are two main components of HTA: Assessment and appraisal.
- The reciprocal relationship between assessment and appraisal informs decision-making in Health Technology Assessment (HTA).
In some countries, the assessment and appraisal functions of an HTA may be carried out by separate bodies.
- One body may be dedicated to an assessment function – synthesising evidence or critically reviewing evidence submissions.
- Another different body may be dedicated to an appraisal function – considering the assessment in light of wider factors related to the local context. They then provide advice or recommendations.
HTA: Assessment
HTA processes pertaining to medicines typically begin with a company submitting a dossier of relevant information to an HTA body. For interventions other than medicines, HTA bodies usually perform a systematic review of published information. By default, the dossier includes detailed evidence relating to the safety and efficacy of the new technology as well as ‘added clinical benefit’ – in other words a comparison of the clinical effectiveness of the new product with the existing standard of care (the comparator).
Some HTA systems in Europe also estimate the impact the new product may have on the health system’s budget (a budget impact evaluation) or the effectiveness of the medicine in comparison to its costs to the system (for instance, a cost-effectiveness analysis or economic evaluation). Not all HTA systems in Europe place the same emphasis on comparative cost-effectiveness analysis, but all focus on the added clinical benefit.
The most common components of an application dossier or ‘submission’ are listed below: note that some of these components are more quantitative than others. Equity, legal, and public health aspects may be more qualitative and therefore may be included in the appraisal part of HTA rather than the evaluation part.
- Target patient population: The specific population that is to be considered for coverage (determined by the full licensed indication or a sub-group within that).
- Disease burden: Also known as ‘unmet need’ or ‘therapeutic need’. This may be a measure of the number of people affected by a particular disease for whom current treatments are inadequate. It may include the number of new diagnoses of a disease, or the costs to society or a government representing those affected. It may also include more qualitative aspects about the burden of disease and current treatments available to patients.
- Medicine description: A description of the medicine, how it works, method of delivery (e.g. injection, tablet), where it is administered to patients, (e.g. in hospital, community, primary care, at home), how often, and its appropriate use in therapy along with other interventions and medicines.
- Clinical efficacy: In medicine, clinical efficacy indicates a positive therapeutic effect. If efficacy is established, an intervention is likely to be at least as good as other available interventions to which it will have been compared.
When talking in terms of efficacy versus effectiveness, efficacy measures how well a treatment works in clinical trials or laboratory studies. Effectiveness, on the other hand, relates to how well a treatment works in the practice of medicine. - Relative efficacy: This is the extent to which an intervention does more good than harm under ideal circumstances compared to one or more alternative interventions.
- Clinical effectiveness: Clinical effectiveness is a measure of how well a particular treatment works in the practice of medicine. It depends on the application of the best knowledge derived from research, clinical experience, and patient preferences.
- Relative clinical effectiveness: This can be defined as the extent to which an intervention does more good than harm compared to one or more intervention alternatives for achieving the desired results when provided under the usual circumstances of health care practice.
- Economic evaluation and cost-effectiveness: In the context of pharmacoeconomics, cost effectiveness is studied by looking at the results of different interventions by measuring a single outcome, usually in 'natural' units (for example, life-years gained, deaths avoided, heart attacks avoided, or cases detected).
Alternative interventions are then compared in terms of cost per (natural) unit of effectiveness in order to assess how it provides value for money. This helps decision-makers determine where to allocate limited healthcare resources.
Cost effectiveness, however, is only one of a number of criteria that should be used to determine whether or not interventions are made available. Other issues, such as equity, needs, impact on working life, and patient priorities should also be part of the economic evaluation. - Budget impact: The costs within a particular timeframe and related to a particular healthcare budget rather than a country’s overall budget. This assumes robust data on epidemiology and treatment patterns, along with assumptions of uptake and displacement of current treatments.
- Innovative characteristics: An assessment of whether there are advantages to using the medicine beyond the added clinical benefit (such as convenience to patients of, for example, a different mode of delivery, or other characteristics that may improve adherence to therapy, with resulting improvements in clinical outcomes and / or quality of life).
- Availability of therapeutic alternatives: A description of what else is available to treat the disease. This may or may not be another medicine.
- Equity considerations: An assessment of how adoption of the new therapy might impact measures of fairness within the health system. For example, will the therapy lead to more benefits for people who are socially or economically disadvantaged?
- Public health impact: An examination of how the new therapy might have a broader impact on public health. For example, a new therapy to treat HIV/AIDS may reduce the rate of HIV transmission within a community.
Most HTA bodies have developed guidelines for companies in order to make this process consistent and create fair comparisons. However, guidelines vary from country to country, and may be available on the websites of most HTA bodies and can help explain how decisions about new medicines are made.
Dossiers are scrutinised by HTA bodies either directly or using academic affiliates. Some HTA bodies conduct independent reviews of the clinical and the economic evidence in order to reduce conflicts of interest.
HTA: Appraisal
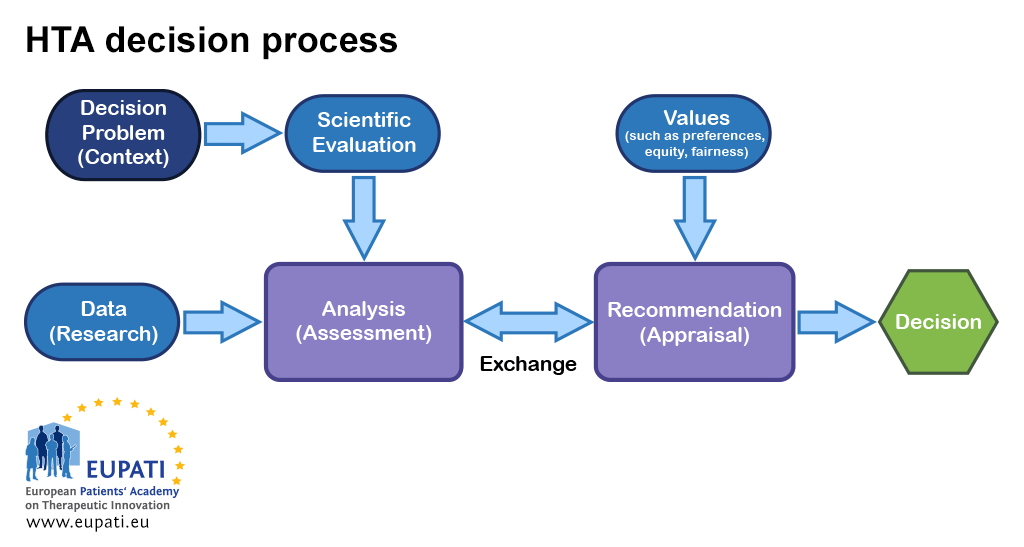
As decision-making regarding reimbursement of a new health technology can be controversial, the best practice approach is to separate evidence assessment from appraisal and also from decision-making. Typically, the bodies that conduct an appraisal will base their recommendations on the outcome of the evidence assessment as well as additional inputs, such as local health policies, values, and patient testimony.
HTA processes generally result in a decision to list or not to list the new technology for reimbursement in an insurance-based system (the list includes medicinal products with reimbursement from the public health insurance), or recommend it for use in a taxation-based national health service. This may be a listing/recommendation for use of the medicine under restricted conditions, for example, for a smaller population of patients with more severe illness.
- Various inputs are relevant at different points in the HTA decision process.
Determining whether an intervention will reduce heart attack rates, cause significant side effects, or increase costs requires judgements about the robustness of evidence. There are always uncertainties in the evidence. Clearly, it is in the best interests of any HTA body to use sound scientific judgment and consistent, transparent approaches that lead to defensible decisions. Given the multidisciplinary nature of HTA, the best approaches from epidemiology, sociology, economics, ethics, law, etc. to support the various analyses are required.
Making a decision, however, requires recognition of what society and patients value. Is it a good thing to reduce heart attack rates? At what cost?
Good approaches to appraisals will involve multiple perspectives and therefore cannot satisfactorily be undertaken by a single individual. For this reason, a committee is convened that uses an explicit and transparent process to arrive at a recommendation. This process is often called deliberative appraisal. Most HTA bodies place greater emphasis on the magnitude of (and strength of evidence for) gains in patient-relevant health outcomes seen in well-designed clinical trials with appropriate comparators.
The next most important aspect is often one or more economic considerations. Almost all HTA agencies consider budget impact (the total amount that the use of the new medicine will add to the health system budget over a defined period). This should be a net budget figure: one that deducts the savings that might occur elsewhere in the health system as a result of benefits associated with the new medicine (for example, fewer hospital admissions due to severe adverse events). The neutrality of the committee structure must be ensured – in other words, members of the committee must formally declare any possible conflicts of interest or decline their participation.
Some HTA bodies have adopted an ethical framework that allows for their recommendations to be reviewed by a broader set of stakeholders. This lets companies, clinicians, or patients who may be unjustly impacted by a flawed, biased, or imprecise recommendation to make an appeal.
Rarely, HTA bodies seek citizens’ views about challenging aspects of decision-making when deciding priorities in healthcare. For example, in the UK NICE has a Citizens’ Council that uses a citizens’ jury approach to provide social value judgements that can inform the NICE appraisal committees. The list below details some of the issues that the Citizens’ Council has advised on.
| Year | Topic |
|---|---|
| 2002 | Clinical need |
| 2003 | Age and cost-effectiveness |
| 2004 | Ultra-orphan drugs and cost-effectiveness |
| 2005 | Mandatory public health measures |
| 2006 | Use of the rule of rescue |
| 2007 | Patient safety and cost-effectiveness |
| 2008 | Departing from the ICER threshold |
| 2009 | Innovation |
| 2010 | Health improvement and financial incentives |
| 2011 | Discounting costs and benefits |
| 2012 | Social care values |
In some cases, HTA outcomes will be linked to price negotiations. Negotiating price is one mechanism for governments to provide access to new therapies (that is, finding a way not to say ‘no’). Other variables include restrictions on who may be able to receive the treatment under reimbursement mechanisms.
Beyond recommendations
Recommendations about whether or not a new medicine can be made available within a healthcare system may be considered to be too rigid and not offering flexibility by those who require access to new therapies. Since these recommendations are generally population-focused, they may not allow for exceptions on an individual basis. Rather than a yes/no recommendation, other mechanisms have been applied by HTA that may be more helpful.
- Coverage with evidence development (CED): This can be used to allow access to a promising new medicine which, at present, has insufficient data supporting either clinical or cost effectiveness. In these circumstances, HTA can recommend use of the medicine, providing there is a formal collection of evidence to resolve those uncertainties while it is being used, for example in a registry. Alternatively, there may be ongoing clinical trials required by regulatory authorities that will deliver additional evidence at some point in the future.
- Price determination: The price of a health technology can have a direct effect on providers and patients’ access to that technology. In some instances, payers may negotiate with the company for a price based on the perceived value of the health technology, especially when the health technology is useful in some cases but not in all. This approach ensures that those providers and patients who need a certain technology have access to it. HTA bodies may or may not be involved in this process. However, value-based pricing presents challenges, as it is difficult to ensure that all aspects of a health technology’s value are adequately considered. For instance, the results of short-term clinical trials may not show the product features that are valuable to patients such as convenience of dosage schedules or less-invasive methods of delivery.
- Decision aids and clinical guidelines: The HTA may indicate that the medicine has most value when used in a particular group of patients or in a particular sequence following other treatment options. To optimise the value, the payer may decide to reimburse the medicine in association with specific clinical guidelines (for prescribers) or specific decision aids (for patients and clinicians). Decision aids are tools for patients and doctors to use evidence to inform an individual decision. They help patients choose between two treatments that have different risks and benefits. It enables them to have more informed discussions with their doctors about what they value most and to determine which the best option for them is.1
- Health system priority setting and budgets: Methods have evolved to use HTA information to determine what services should be paid for (e.g. to determine what services should be included in universal health coverage). That is, what is the optimal mix that provides value and is affordable to the payer.2
HTA networks
Many HTA organisations in Europe are also linked together by the European Union Network of HTA organisations (EUnetHTA) formed in 2004. EUnetHTA works closely with the EU Commission, the European Medicines Agency (EMA) and stakeholder organisations representing patients/consumers, industry, payers (statutory health insurance), and healthcare providers. EUnetHTA is working to develop the methods, standards, and processes of the Network for HTA in Europe (HTA Network).
The HTA Network will promote good HTA practices and methods in response to the high level of diversity in European HTA methods, practices, and outcomes, as well as the high level of duplication of effort. It will also aim to facilitate efficient use of HTA resources in Europe. Key activities that EUnetHTA are undertaking for the HTA Network include the development of HTA methodology guidelines and piloting of joint assessments of relative effectiveness. These activities will help reduce the level of workload at the national level and make it easier for HTA bodies at the Member State level to conduct the additional analyses and decision making that are specific to their health system.
Further Resources[glossary_exclude]
- Health Technology Assessment Network. Retrieved 6 July, 2021, from https://ec.europa.eu/health/technology_assessment/policy/network_en
- EUnetHTA: Retrieved 6 January, 2016, http://www.eunethta.eu/
- Opportunities for patients to be involved with EUnetHTA: Retrieved 6 January, 2016, http://www.eunethta.eu/
- Sorenson, C., Drummond, M., and Panos, K. (2008). Ensuring value for money in health care: The role of health technology assessment in the European Union. Copenhagen: World Health Organization. Retrieved 6 January, 2016, from http://www.euro.who.int/__data/assets/pdf_file/0011/98291/E91271.pdf
- Velasco Garrido, M., Kristensen, F.B., Nielsen, C.P, and Busse, R. (2008). Health technology assessment and health policy-making in Europe: Current status, challenges and potential. Copenhagen: World Health Organization. Retrieved 6 January, 2016, from http://www.euro.who.int/__data/assets/pdf_file/0003/90426/E91922.pdf
- Kleinjen, S., George, E., Goulden, S., et al. (2012). ‘Relative effectiveness assessment of pharmaceuticals: similarities and differences in 29 jurisdictions’. Value Health, (15), 954-960. Retrieved 6 January, 2016, from http://www.valueinhealthjournal.com/article/S1098-3015(12)01609-9/pdf
- Rawlins, M. (2014). ‘Evidence, values, and decision-making.’ International Journal of Technology Assessment in Health Care, (30), 233-238.
References
- Ottawa Hospital Research Institute (2014). Patient Decision Aids: Implementation Toolkit. Retrieved 6 January, 2016, from http://decisionaid.ohri.ca/implement.html
- Bandolier (2007). Programme budgeting and marginal analysis. Retrieved 6 July, 2021 from http://www.bandolier.org.uk/booth/glossary/PBMA.html[/glossary_exclude]
A2-6.05-v1.1