Evidence-based medicine
Introduction
Lisa has serious pain following surgery. The doctor has to choose between tablets, according to external clinical evidence, and injection according to personal clinical experience and patient preferences. The doctor knows that according to external clinical evidence, tablets containing morphine would be the best choice. However, a common side effect of the anaesthesia given to Lisa during surgery is vomiting. This means that if Lisa is given a tablet and starts vomiting, the tablet will be brought up and she will get no pain relieving effect. The doctor and Lisa know from experience that Lisa is likely to start vomiting within 30 minutes after anaesthesia has ended. Therefore, the doctor decides to give Lisa an injection containing morphine instead.
In the example, the doctor decides, based on personal clinical experience and patient preferences, to use a morphine injection instead of tablets containing morphine, which have the best external clinical evidence. The doctor uses the same medical compound (i.e. morphine), as suggested in the external clinical evidence, but chooses to use a different formulation (i.e. injection instead of tablet).
This is an example of how a doctor comes to a specific treatment decision based on evidence following a discussion with the patient.
What is Evidence-based medicine?
Evidence-based medicine (EBM) is the process of systematically reviewing, appraising, and using clinical research findings to aid the delivery of optimum clinical care to patients. Patient knowledge of evidence-based medicine is important because it enables them to make more informed decisions about disease management and treatment. It also gives patients a more accurate perception of risk, encourages appropriate use of elective procedures, and supports evidence-based doctor/patient decision-making.
Evidence-based medicine is a combination of principles and methods. When put into action, these ensure that medical decisions, guidelines, and policies are based on the current best evidence about the effects of different forms of treatment and healthcare in general. With respect to medicines, it draws heavily on information from the benefit and risk (efficacy and safety) evaluation.
The concept of evidence-based medicine emerged in the 1950s. Before then, medical decisions were mainly based on medical training, clinical experience, and journal reading. However, studies showed that medical treatment decisions differed significantly between individual healthcare professionals. The basis was formed for implementing systematic methods to collect, evaluate, and organise research data – which lead to evidence-based medicine. Since its implementation, evidence-based medicine has been recognised by doctors, pharmaceutical companies, regulatory authorities, and the general public.
The decision-maker needs to look at knowledge from their own clinical experience along with the best evidence from controlled studies and research. Combining clinical experience and controlled studies in the decision-making process is important. Without clinical experience, the risk related to a given treatment may end up causing unwanted effects.
5-step model of evidence-based medicine
One approach to evidence-based medicine is based on a 5 step model:
- Defining a clinically relevant question (doctor searches for information to find correct diagnosis)
- Searching for the best evidence (doctor searches for evidence to support the findings from Step 1)
- Assessing the quality of the evidence (doctor ensures that quality and reliability is high)
- Acting on the evidence to form a clinical decision (based on Steps 1-3, patient and doctor jointly make an informed treatment decision)
- Evaluating the process (doctor and patient assess if the intended outcome is achieved and adjust treatment decisions accordingly if needed)
With reference to the example in the beginning, the doctor’s choice is in line with the principles of evidence-based medicine as well as patient’s feedback. The doctor’s decision includes conscientious, explicit, and judicious use of the best evidence at the current time point, including the patient’s experience, when deciding how to provide the best possible medical treatment for a given patient.
Patient engagements in decision-making processes have an important role to play in building new guidelines of treatment principles. This includes reading, understanding, and acting on health information; working together with clinicians to evaluate and select the right treatment options; and providing feedback on outcomes. Patients can have an active role to play at all levels of evidence.
Assessing evidence for evidence-based medicine
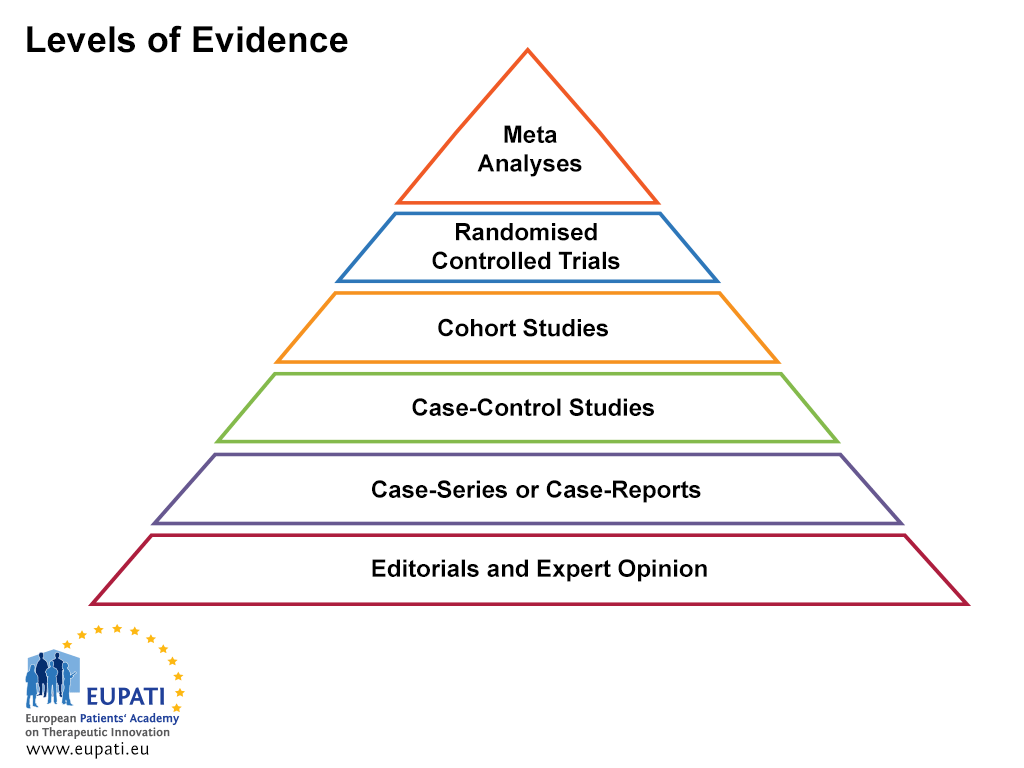
To assess the quality of evidence, the information collected is ranked according to the different levels of evidence. The pyramid in the figure below shows the various levels of evidence and their relative rankings.
- Levels of evidence are useful when assessing the quality of evidence.
Editorial and expert opinions
This is evidence based on the opinions of a panel of experts aiming to shape common medical practice.
Case series and case reports
Case series are descriptive studies following one small group of people. They are additions or supplements of case reports. A case report is a detailed report of the symptoms, signs, diagnosis, treatment, and follow-up of an individual patient.
Case-control study
A case-control study is an observational retrospective study (looking at historical data) that compares patients who have a disease with patients who do not have the disease. Outcomes such as lung cancer are generally studied by the use of case-control studies. A group of smokers (the exposed group) and a group of non-smokers (the unexposed group) are recruited and followed over time. The differences in the incidence of lung cancer between the groups are then documented, allowing the variable being assessed (the ‘independent variable – in this case, smoking), to be isolated as the cause of the ‘dependent variable’ (in this case, lung cancer).
In this example, a statistically significant increase in the incidence of lung cancer in the smoking group as compared to the non-smoking group would be considered as evidence in favour of assuming a causal relationship between smoking and lung cancer.
Cohort study
The modern definition of a ‘cohort’ in clinical studies is a group of people with defined characteristics who are followed in order to determine health-related outcomes.
The Framingham Heart study is an example of the use of a cohort study to answer an epidemiological question. The Framingham study began in 1948 and is still ongoing. The objective is to study the impact of several factors on the incidence of heart diseases. The question that the study aims to address is: Do factors such as high blood pressure, smoking, high body weight, diabetes, exercise, etc., have a link to the development of heart disease? In order to investigate each exposure (for instance, smoking), the investigators would recruit a cohort of smokers (the exposed group) and a cohort of non-smokers (the unexposed group). The cohorts would then be followed for a set period of time. Differences in the incidence of heart disease between the cohorts at the end of this time are then documented. The cohorts are matched in terms of many other variables, such as:
- Economic status (for instance education, income, and occupation)
- Health status (for instance the presence of other diseases)
This means that the variable being assessed, the ‘independent variable’ (in this case, smoking), can be isolated as the cause of the ‘dependent variable’ (in this case, heart disease).
In this example, a statistically significant increase in the incidence of heart diseases in the smoking group as compared to the non-smoking group is evidence in favour of assuming a causal relationship between smoking and the development of heart disease. The findings of the Framingham Heart Study have, over the years, provided conclusive evidence that cardiovascular diseases are largely the result of measurable and modifiable risk factors, and that individuals can gain control over their heart health by: looking carefully at their diet and lifestyle and changing their intake of saturated fat, cholesterol, and smoking; losing weight or becoming physically active; and regulating their levels of stress and blood pressure. It is principally because of the Framingham Heart Study we now have a good understanding of the relationship of certain risk factors to heart disease.
Another example of a cohort study that has been ongoing for many years is the National Child Development Study (NCDS), the most widely-researched of the British birth cohort studies. The largest study in women is the Nurses Health Study. This study began in 1976 and is tracking over 120,000. Data from this study has been analysed for many different conditions and outcomes.
Randomised clinical trial
A randomised clinical trial is one that uses randomisation when allocating people to different arms of the study. This means that the treatment groups are chosen by chance using a formal system and each participant has an equal chance of being selected to each arm.
Meta-analysis
A meta-analysis is a systematic, statistics-based review of data that contrasts and combines results from different but related studies, in attempt to identify patterns, disagreements, and other relationships across multiple studies. A meta-analysis can support a stronger conclusion than any individual study, but may be flawed because of publication bias.
Outcomes research
Outcomes research is a broad umbrella term without a consistent definition. In brief, outcomes research studies the end results of medical care – the effect of the healthcare process on the health and well-being of patients. In other words, clinical outcomes research seeks to monitor, understand, and improve the impact of medical treatment on a specific patient or population. It tends to describe research that is concerned with the effectiveness of public-health interventions and health services; that is, the outcomes of these services.
Attention is frequently focused on the affected individual – in other words, the clinical endpoints (overall outcomes) most relevant to the patient or population. Such endpoints could be quality of life or pain level. However, outcomes research may also focus on the effectiveness of healthcare delivery, with measures such as cost-effectiveness, health status, and disease burden (the impact of the health problem).
The difference between EBM and outcomes research is one of focus: While the main focus of EBM is providing the best care to the patient according to clinical evidence and experience, the main focus of outcome research is predefined endpoints. In clinical outcome research, these endpoints are usually clinically relevant endpoints.
| Type of endpoint | Example |
|---|---|
| Physiological measure (biomarker) | Blood pressure |
| Clinical | Heart pressure |
| Symptoms | Coughing |
| Functional and care | Measurement of function, for instance ability to perform tasks of everyday living, Quality of Life assessments |
In outcomes research, the relevant endpoints are often symptoms or functional and care measures – things considered important by the patient receiving the treatment. For instance, a patient suffering an infection who is then given penicillin may be more concerned with no longer having a fever and feeling better than they are concerned with the effect of the penicillin on the actual levels of infection. In this case, their symptoms and the way they feel are considered a direct assessment of their health status – in other words, the endpoints that outcomes research would focus on. The patient is also likely to be interested in the potential side effects associated with penicillin, as well as the cost of the treatment. For other diseases, such as cancer, an important clinical outcome relevant for the patient is the risk of dying.
Where the duration of the study is long, outcomes research studies can include the use of ‘surrogate endpoints’. A surrogate endpoint is when a biomarker is used to measure an outcome – it acts as a substitute for a clinical efficacy endpoint. Consider a clinical study where the effect of penicillin treatment is measured by a decrease in the amount of a specific protein (called the ‘C-reactive protein’), which is always present in the blood. In a healthy person, the amount of this protein in the blood is very small, but it increases dramatically upon acute infection. Measuring the levels of C-reactive protein is therefore an indirect way of measuring infection in the body – in this case, the protein serves as a ‘biomarker’ for infection. A biomarker is a measurable indicator of a disease state. This then correlates with the risk or progress of a disease, or with how the disease is likely to respond to a given treatment. In daily practice, a blood sample is taken from the patient and the amount of the biomarker in the blood is measured.
It has to be underlined that for a surrogate endpoint to be used for regulatory purposes, the marker should have previously been confirmed or validated. It must be shown that changes in the biomarker correlate (correspond) with the clinical outcome of a specific disease and the treatment effect.
Further Resources
- World Health Organisation (2008). Where are the patients in decision-making about their own care? Retrieved 31 August, 2015.
Attachments
- Presentation: Evidence-based Medicine
Size: 454,790 bytes, Format: .pptx
A presentation describing evidence based medicine, which can be adapted for own use.
A2-1.10-v1.3