Clinical study results: Publication
Introduction
Clinical trials make new medicines and improved treatments available for patients. The information that clinical trials generate on the efficacy and safety of these treatments is important for patients and their physicians to make informed treatment decisions. The utility of a treatment must be assessed globally, taking all the results available from clinical studies investigating the treatment. Access to information on clinical trials is one important means for improving the efficiency in research by reducing the duplication or replication of research efforts. The transparency of clinical trial information is important in ensuring trust in clinical study results. The reader will have to critically review the information published on clinical trials.
What are clinical study results?
The results of a clinical study or trial are all the data, measures, and statistical analyses generated during that clinical study.
Study results include the following elements:
- Description of study population: The number of participants per study treatment arm who started, completed, and dropped out of the study.
- Baseline data: Data collected at the beginning of a clinical study. These data include: demographics (such as age and gender); patient characteristics (such as weight, height, blood pressure, etc.); and study-specific measures (such as disease characteristics or prior treatment).
- Measures capturing the effect of the treatment on participants: For example, medicine activity in a Phase II trial, patient survival, and/or quality of life in Phase III trials.
- Adverse events experienced by the study participants: For example, pain, nausea, and other side effects.
The Clinical Study Report (CSR) is the formal document describing the results of a clinical study and provides evidence for its use in humans. CSRs follow a format laid down by the regulatory authorities. The CSR is prepared by the study sponsor and forms a part of the Common Technical Document (CTD). Access to clinical study reports is usually limited to the sponsor and the regulatory authorities assessing a Marketing Authorisation Application (MAA), due to confidential and commercial issues.
Publication of clinical study results
At the end of the clinical study and its analysis, researchers may present their conclusions at scientific meetings and in medical journals. Before publication in medical journals, the manuscript is peer-reviewed by independent experts appointed by the journal editor.
Publications should contain enough details to enable the reader to make their own judgement about the study findings. The confidence that a reader has in the validity of the results is influenced by the quality of the publication. Therefore, various guidelines and checklists are available to guide the reporting of results in a standardised way, depending on the type of research being performed.
Various organisations are currently engaged in initiatives to encourage or require the registration and disclosure of clinical trial information. In Europe, EudraCT, the European Clinical Trials Database of the European Medical Agency (EMA) collects information on all clinical trials of medicines performed in Europe. As of July 2014, this database also makes trial summary results available to the public. For trials taking place in the EU starting after January 1st, 2015, the results must be published – whether they are negative or positive. The World Health Organisation (WHO), through its International Clinical Trials Registry Platform (ICTRP), is setting international standards for registering and reporting on all clinical trials. In the US, the registry clinicaltrials.gov is taking a similar approach.
Levels of evidence in clinical study results
Medical treatment decisions are now largely based on evidence-based medicine (EBM). EBM combines clinical experience with the current best evidence from controlled studies and research to provide the best treatment for a patient. Information on treatment safety and efficacy is important in EBM so that patients and their physicians can make informed treatment decisions.
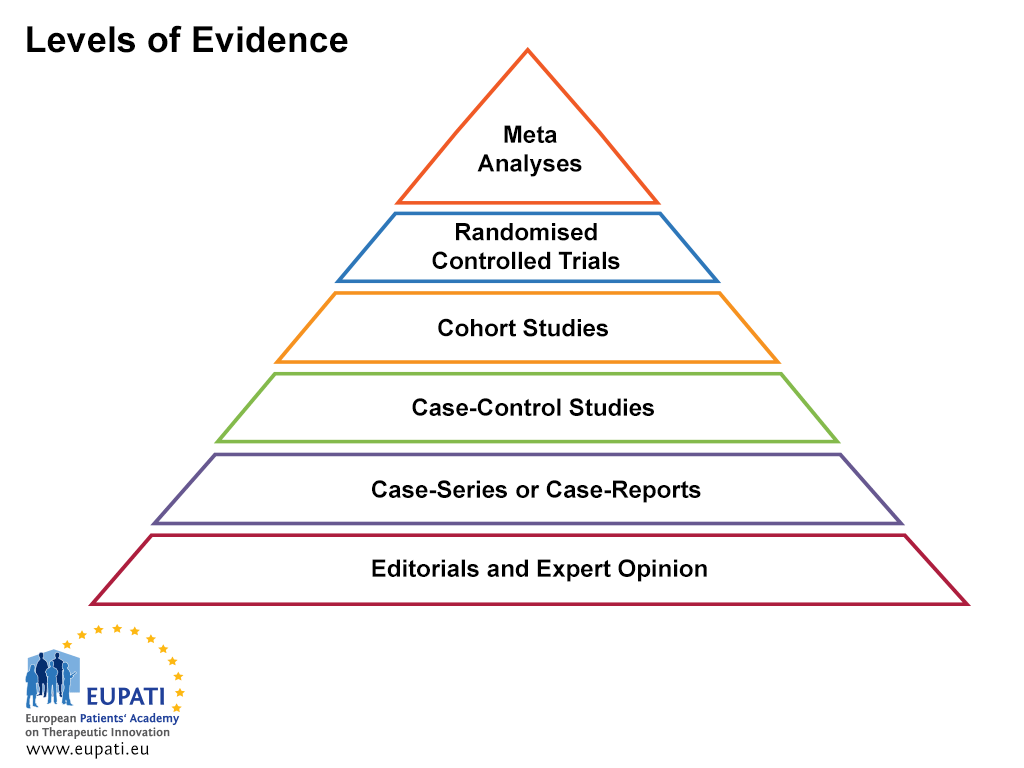
EBM relies on knowledge and review of the current best evidence about the effects of different forms of treatment and healthcare in general. It is important not to limit the search for evidence about a treatment to a single publication. When comparing results coming from different sources, it is important to bear in mind that there are different levels of evidence (see Figure 1 below). Levels of evidence represent and classify the quality of the study and therefore the strength of the evidence that the study provides. Randomised, controlled, blinded studies provide the best scientific evidence of benefit and risk, but are not always available. A meta-analysis, which is a statistics-based review that contrasts and combines results from different but related studies, attempts to identify patterns, disagreements, and other relationships across multiple studies. A meta-analysis can support a stronger conclusion than any individual study, but may be flawed because of publication bias.
- Levels of evidence are useful when assessing the quality of evidence.
In general, the types of studies are:
- Adequately powered, high-quality randomised trial, or meta-analysis of randomised trials showing statistically consistent results
- Randomised trials inadequately powered, possibly biased, or showing statistically inconsistent results
- Non-randomised studies with concurrent controls
- Non-randomised studies with historical controls (for instance, a typical single-arm Phase II study)
- Expert committee review, case reports, retrospective studies
Sources of errors in publications
The three most common sources of errors in publications are:1
- The risk of misuse and misrepresentation of statistical tests and their outcomes, due to the confusion about the meaning of numbers (estimates) and the interpretation of hypothesis tests (p-values, power).
- Data dredging or testing large numbers of hypotheses in a single data set in the search for a positive effect. When numerous hypotheses are tested with a single data set, it is virtually certain that some hypotheses will appear falsely statistically significant, even though the correlations may not exist in reality. If researchers using data-mining techniques are not cautious, they can be easily misled by these apparently significant results.
- Bias. In research, bias occurs when systematic error is introduced into data sampling or hypothesis testing by selecting or encouraging one outcome or answer over others. Bias is not always the result of intentional actions – it can also be unintentionally introduced.
References
- Goldacre, B. (2010) Bad science: Quacks, hacks, and big pharma flacks. New York: Faber and Faber.
- Rennie, D., & Guyatt, G. (2002). Users' guides to the medical literature: A manual for evidence-based clinical practice. Chicago, IL: American Medical Association.
Attachments
- Presentation: Clinical Study Results Publication
Size: 449,072 bytes, Format: .pptx
A presentation describing the clinical study results publication, which can be adapted for own use.
A2-4.35.1-v1.2