Bioavailability and bioequivalence
Bioavailability
Bioavailability is defined as: the fraction (percentage) of an administered dose of unchanged medicine that reaches the blood stream (systemic circulation).
In all cases when using a medicine, you want the active substance of the medicine, also referred to as the ‘active pharmaceutical ingredient’ (API), to be able to enter the body. However, to have a therapeutic effect, it is not sufficient for the active substance to enter the body. The active substance needs to be available in the correct dose at the specific site in the body where it has to work. This specific site is referred to as the ‘target site’. Also, the active substance needs to reach the target site within a certain time, and be available there for a defined time.
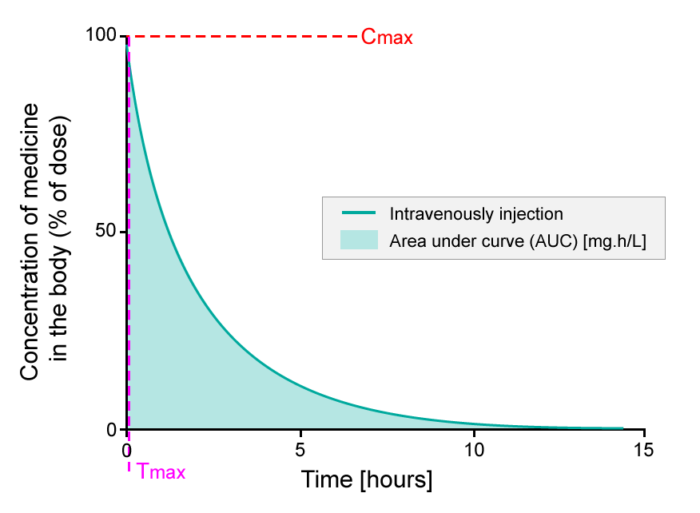
When giving an injection directly into the bloodstream, i.e.an intravenous injection (IV), the bioavailability is defined as 100% (see figure below).
- The percentage of active substance in the body or bioavailability, after direct injection into the bloodstream, studied over a period of 15 hours. The area under the curve (AUC) is shaded. Tmax is the time where the highest concentration of the medicine is found in the blood; Cmax is the maximum concentration of the medicine found in the blood.
Upon injection, an active substance reaches the target site after a complex journey in the bloodstream. When evaluating bioavailability, blood samples are collected, and the concentration of the active substance in the blood (systemic circulation) is determined. Thus, bioavailability will be 100% directly after injection, as the active substance is administered directly into the blood. This is exactly what you see on the y-axis in the figure above (Intravenous bioavailability). So if 75 milligram (mg) of active substance is injected into the bloodstream, then 100% corresponds to 75 mg active substance.
When the active substance circulates via the bloodstream, a fraction of the active substance will be metabolized or excreted, and consequently the concentration of the active substance in the body will decrease over time (see figure above). The bioavailability profile is evaluated and compared to other medicines by looking at the area under the curve (AUC), which represents the total exposure to an active substance that the body receives. The time where the highest concentration of the active substance is found in the blood is called Tmax, and the maximum concentration of the active substance found in the blood stream is called Cmax.
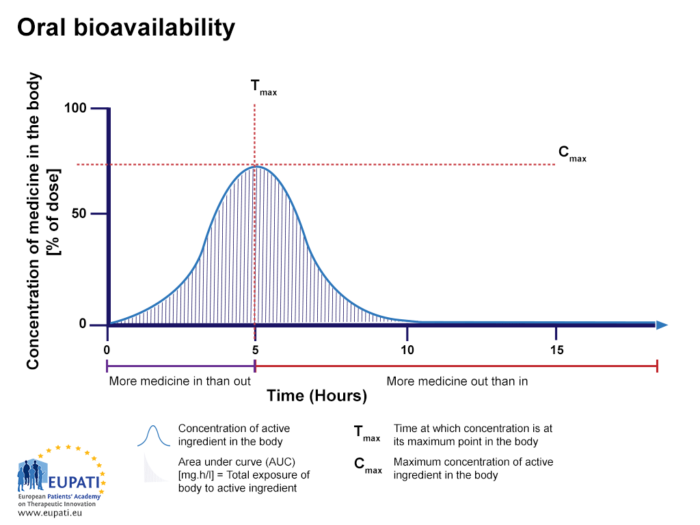
If the same active substance shown in the figure above is given via another route, such as a tablet given orally (by mouth), the bioavailability is lower than 100% (see figure below, Oral bioavailability).
- The percentage of active substance after a tablet is swallowed, studied over the period of 15 hours. The area under the curve (AUC) is shaded and represents the total amount of active substance which was in the bloodstream over the period studied. Tmax is the time where the highest concentration of the medicine is found in the bloodstream, whereas Cmax is the maximum concentration of the medicine found in the bloodstream.
Oral bioavailability
The percentage of active substance after a tablet is swallowed, studied over the period of 15 hours. The AUC is shaded. Tmax is the time where the highest concentration of the medicine is found in the bloodstream, whereas Cmax is the maximum concentration of the medicine found in the bloodstream.
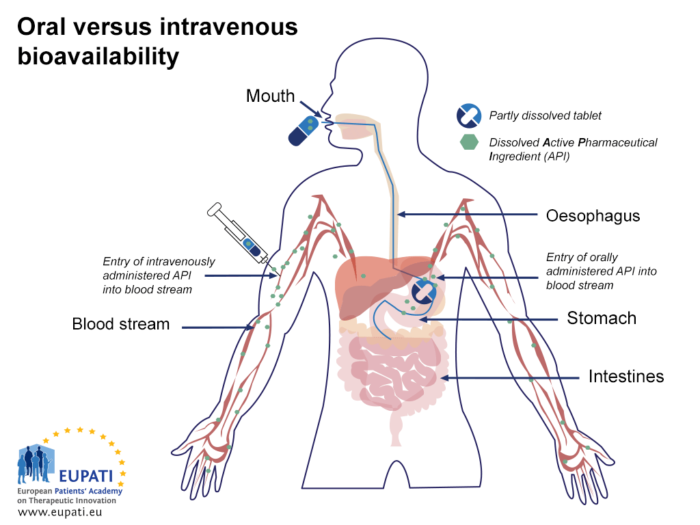
The lower bioavailability of the oral route compared to intravenous injection is explained in the figure below (Oral vs. intravenous bioavailability):
- Comparing the bioavailability of Active Pharmaceutical Ingredients administered orally and intravenously. An API is said to be ‘biologically available’ (bioavailable) when it enters the blood stream.
- Comparing the bioavailability of Active Pharmaceutical Ingredients administered orally and intravenously. An API is said to be ‘biologically available’ (bioavailable) when it enters the blood stream.
After a tablet or capsule is swallowed, it reaches the stomach within a minute or two.1 In the stomach, the tablet or capsule is dissolved, and some of the active substance is absorbed into the bloodstream. The components are transported to the small intestine where absorption is completed. Absorption from the gastrointestinal system can vary greatly. Lower bioavailability can be the result of poor or no absorption from the stomach and the intestines, so this step is an important one that can influence availability.
When the active substance is absorbed, it reaches the hepatic portal vein first, and is transported to the liver. This is the first time the active substance is metabolised in the liver, referred to as the ‘first pass metabolism’. Some active substances are metabolised to a larger extent than others during this first time metabolism. The non-metabolised part of the active substance, normally less than 100%, will reach systematic circulation via the hepatic vein. The amount that actually reaches systemic circulation is referred to as the ‘absolute bioavailability’.
Absolute bioavailability compares the bioavailability of the API in systemic circulation following non-intravenous administration with the bioavailability of the same medicine following intravenous administration. It is the percentage of the API absorbed through non-intravenous administration compared with the corresponding same medicine administered intravenously.
Briefly, in absolute bioavailability the standard is always IV.
Relative bioavailability measures the bioavailability of a formulation (A) of a certain medicine when compared with another formulation (B) of the same medicine, usually an established standard other than IV, or through administration via a different route.
Bioavailability is affected by a number of other factors, which are particular to any one individual. See the attached fact sheet for some examples of bioavailability.
Bioequivalence
Bioequivalence is the relationship between two preparations of the same drug in the same dosage form that have a similar bioavailability.
The relative bioavailability is used not only to compare different formulations, but also when two tablets (or any other medicines with same formulation) with the same active substance from different pharmaceutical companies need to be compared. The tablet from company A is referred to as a generic medicine relative to the reference tablet from company B (branded medicine). To see whether tablet A is bioequivalent to tablet B, the bioavailability rates of the two are compared.2
[glossary_exclude]Further Resources
- Food and Drug Administation (2002). Guidance for industry: Bioavailability and bioequivalence studies for orally administered drug products – General considerations. Rockville, MD: Food and Drug Administration. Retrieved 23 June, 2015, from http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/UCM154838.pdf
- Wang, H., Li, Q., Reyes, S., Zhang, J., Xie, L., Melendez, V., Hickman, M. and Kozar, M.P. (2013). Formulation and particle size reduction improve bioavailability of poorly water-soluble compounds with antimalarial activity. Malaria Research and Treatment, Retrieved June 23, 2015, from http://dx.doi.org/10.1155/2013/769234
- Johnson, J.A. (2000). Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. International Journal of Clinical Pharmacology and Therapeutics, 38, 53-60.[/glossary_exclude]
[glossary_exclude]References
- Tatum, R.P., Shi, G., Manka, M.A., Brasseur, J.G., Joehl, R.J. and Kahrilas, P.J. (2000). Bolus transit assessed by an esophageal stress test in postfundoplication dysphagia. Journal of Surgical Research, 91, 56–60.
- MobiSystems, Inc. (2007). Dorland's Medical Dictionary for Health Consumers. [Mobile application software].[/glossary_exclude]
[glossary_exclude]Attachments
- Fact Sheet: Bioavailability Examples
Size: 110,012 bytes, Format: .docx
Examples of bioavailability related to penicillin and asthma.
A2-1.16-V1.2