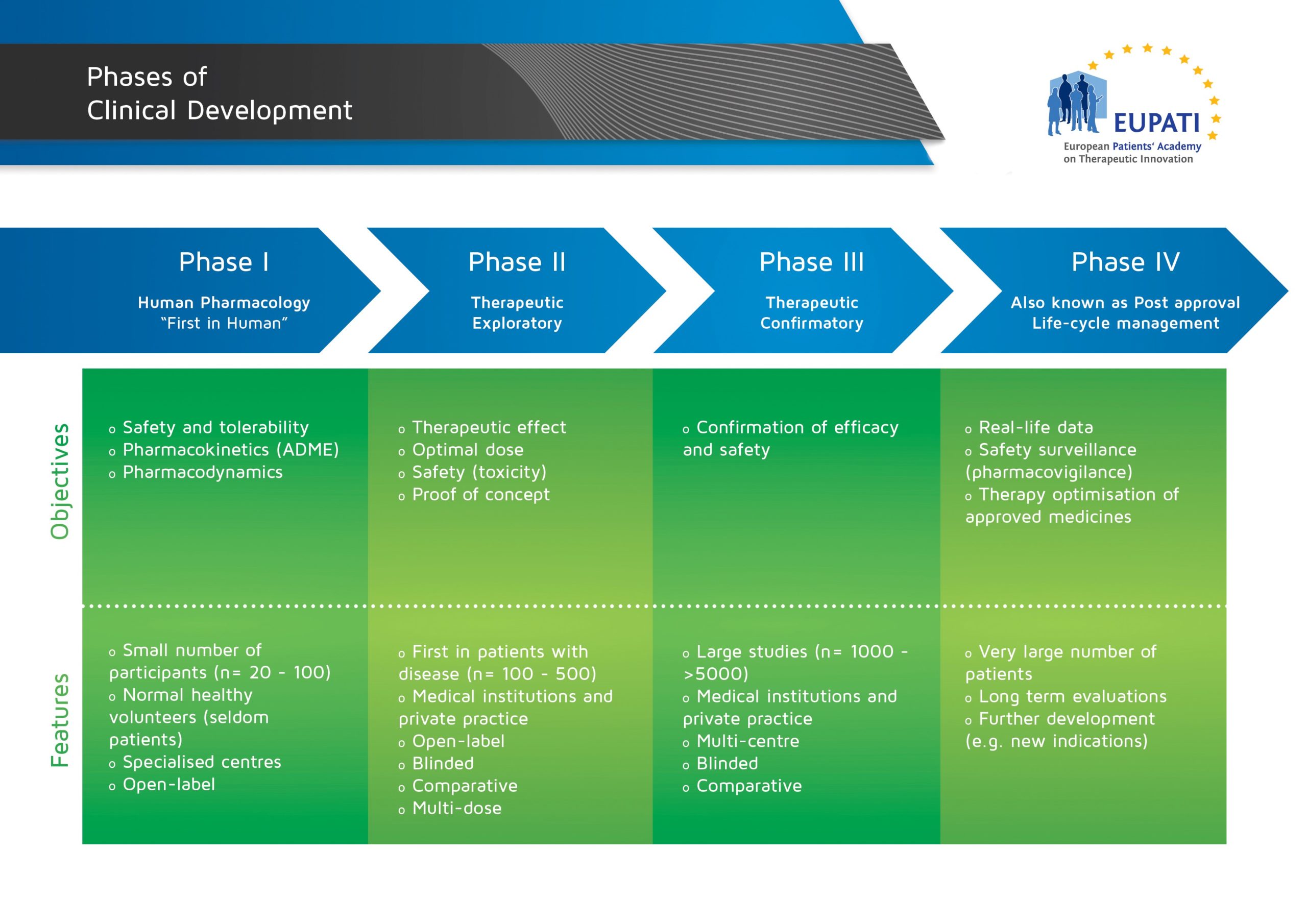
Phase IV Trials
Phase IV trials are usually conducted after marketing authorisation is granted and the medicine is in general use.
Phase IV studies are also known as post-authorisation safety studies (PASS) and may be voluntary or imposed by the regulatory authorities. The possibility also exists of requesting the marketing authorisation holder to conduct post-authorisation efficacy studies (PAESs) in order to complement efficacy data that are available at the time of the initial authorisation. Phase IV studies collect additional information about side-effects and safety, long-term risks and benefits, and/or how well the medicine works when used widely.
- The four phases of clinical development differ in terms of their objectives and features.